Abeta-degrading enzymes in Alzheimer's disease
- PMID: 18363935
- PMCID: PMC8095507
- DOI: 10.1111/j.1750-3639.2008.00132.x
Abeta-degrading enzymes in Alzheimer's disease
Abstract
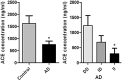
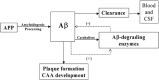
In Alzheimer's disease (AD) Abeta accumulates because of imbalance between the production of Abeta and its removal from the brain. There is increasing evidence that in most sporadic forms of AD, the accumulation of Abeta is partly, if not in some cases solely, because of defects in its removal--mediated through a combination of diffusion along perivascular extracellular matrix, transport across vessel walls into the blood stream and enzymatic degradation. Multiple enzymes within the central nervous system (CNS) are capable of degrading Abeta. Most are produced by neurons or glia, but some are expressed in the cerebral vasculature, where reduced Abeta-degrading activity may contribute to the development of cerebral amyloid angiopathy (CAA). Neprilysin and insulin-degrading enzyme (IDE), which have been most extensively studied, are expressed both neuronally and within the vasculature. The levels of both of these enzymes are reduced in AD although the correlation with enzyme activity is still not entirely clear. Other enzymes shown capable of degrading Abetain vitro or in animal studies include plasmin; endothelin-converting enzymes ECE-1 and -2; matrix metalloproteinases MMP-2, -3 and -9; and angiotensin-converting enzyme (ACE). The levels of plasmin and plasminogen activators (uPA and tPA) and ECE-2 are reported to be reduced in AD. Reductions in neprilysin, IDE and plasmin in AD have been associated with possession of APOEepsilon4. We found no change in the level or activity of MMP-2, -3 or -9 in AD. The level and activity of ACE are increased, the level being directly related to Abeta plaque load. Up-regulation of some Abeta-degrading enzymes may initially compensate for declining activity of others, but as age, genetic factors and diseases such as hypertension and diabetes diminish the effectiveness of other Abeta-clearance pathways, reductions in the activity of particular Abeta-degrading enzymes may become critical, leading to the development of AD and CAA.
Figures

Similar articles
-
Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain.J Neurochem. 2010 Oct;115(1):47-57. doi: 10.1111/j.1471-4159.2010.06899.x. Epub 2010 Jul 30. J Neurochem. 2010. PMID: 20663017 Free PMC article.
-
Experimental approaches for altering the expression of Abeta-degrading enzymes.J Neurochem. 2023 Mar;164(6):725-763. doi: 10.1111/jnc.15762. Epub 2023 Feb 1. J Neurochem. 2023. PMID: 36633092 Review.
-
Molecular basis of selective amyloid-β degrading enzymes in Alzheimer's disease.FEBS J. 2024 Jul;291(14):2999-3029. doi: 10.1111/febs.16939. Epub 2023 Sep 8. FEBS J. 2024. PMID: 37622248 Review.
-
Abeta-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention.Biochem Soc Trans. 2005 Nov;33(Pt 5):1101-5. doi: 10.1042/BST20051101. Biochem Soc Trans. 2005. PMID: 16246055
-
Angiotensin-converting enzyme as a potential target for treatment of Alzheimer's disease: inhibition or activation?Rev Neurosci. 2008;19(4-5):203-12. doi: 10.1515/revneuro.2008.19.4-5.203. Rev Neurosci. 2008. PMID: 19145983 Review.
Cited by
-
1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer's disease patients.J Alzheimers Dis. 2013;34(1):155-70. doi: 10.3233/JAD-121735. J Alzheimers Dis. 2013. PMID: 23186989 Free PMC article.
-
Systems proteomic analysis reveals that clusterin and tissue inhibitor of metalloproteinases 3 increase in leptomeningeal arteries affected by cerebral amyloid angiopathy.Neuropathol Appl Neurobiol. 2017 Oct;43(6):492-504. doi: 10.1111/nan.12342. Epub 2016 Oct 5. Neuropathol Appl Neurobiol. 2017. PMID: 27543695 Free PMC article.
-
Overexpression of the Insulin-Like Growth Factor II Receptor Increases β-Amyloid Production and Affects Cell Viability.Mol Cell Biol. 2015 Jul;35(14):2368-84. doi: 10.1128/MCB.01338-14. Epub 2015 May 4. Mol Cell Biol. 2015. PMID: 25939386 Free PMC article.
-
Matrix metalloproteinases-2 and -3 are reduced in cerebrospinal fluid with low beta-amyloid1-42 levels.Neurosci Lett. 2009 Dec 11;466(3):135-8. doi: 10.1016/j.neulet.2009.09.043. Epub 2009 Sep 26. Neurosci Lett. 2009. PMID: 19786072 Free PMC article.
-
CALHM1 P86L polymorphism modulates CSF Aβ levels in cognitively healthy individuals at risk for Alzheimer's disease.Mol Med. 2011 Sep-Oct;17(9-10):974-9. doi: 10.2119/molmed.2011.00154. Epub 2011 May 24. Mol Med. 2011. PMID: 21629967 Free PMC article.
References
-
- Abraham R, Myers A, Wavrant‐DeVrieze F, Hamshere ML, Thomas HV, Marshall H et al (2001) Substantial linkage disequilibrium across the insulin‐degrading enzyme locus but no association with late‐onset alzheimer's disease. Hum Genet 109:646–652. - PubMed
-
- Affholter JA, Hsieh CL, Francke U, Roth RA (1990) Insulin‐degrading enzyme: stable expression of the human complementary DNA, characterization of its protein product, and chromosomal mapping of the human and mouse genes. Mol Endocrinol 4:1125–1135. - PubMed
-
- Akiyama H, Shii K, Yokono K, Yonezawa K, Sato S, Watanabe K, Baba S (1988) Cellular localization of insulin‐degrading enzyme in rat liver using monoclonal antibodies specific for this enzyme. Biochem Biophys Res Commun 155:914–922. - PubMed
-
- Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL (2001) Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid beta‐protein (Abeta) deposition. Brain Res 902:277–281. - PubMed
-
- Apelt J, Ach K, Schliebs R (2003) Aging‐related down‐regulation of neprilysin, a putative beta‐amyloid‐degrading enzyme, in transgenic Tg2576 Alzheimer‐like mouse brain is accompanied by an astroglial upregulation in the vicinity of beta‐amyloid plaques. Neurosci Lett 339:183–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

