Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells
- PMID: 18354059
- PMCID: PMC2650426
- DOI: 10.1124/jpet.107.134072
Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells
Abstract
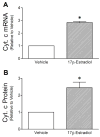
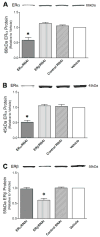
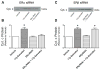
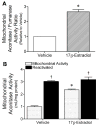
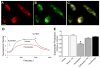
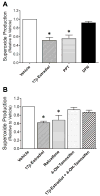
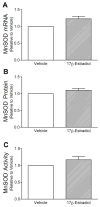
Mitochondrial reactive oxygen species (ROS) and endothelial dysfunction are key contributors to cerebrovascular pathophysiology. We previously found that 17beta-estradiol profoundly affects mitochondrial function in cerebral blood vessels, enhancing efficiency of energy production and suppressing mitochondrial oxidative stress. To determine whether estrogen specifically affects endothelial mitochondria through receptor mechanisms, we used cultured human brain microvascular endothelial cells (HBMECs). 17beta-Estradiol treatment for 24 h increased mitochondrial cytochrome c protein and mRNA; use of silencing RNA for estrogen receptors (ERs) showed that this effect involved ERalpha, but not ERbeta. Mitochondrial ROS were determined by measuring the activity of aconitase, an enzyme with an iron-sulfur center inactivated by mitochondrial superoxide. 17beta-Estradiol increased mitochondrial aconitase activity in HBMECs, indicating a reduction in ROS. Direct measurement of mitochondrial superoxide with MitoSOX Red showed that 17beta-estradiol, but not 17alpha-estradiol, significantly decreased mitochondrial superoxide production, an effect blocked by the ER antagonist, ICI-182,780 (fulvestrant). Selective ER agonists demonstrated that the decrease in mitochondrial superoxide was mediated by ERalpha, not ERbeta. The selective estrogen receptor modulators, raloxifene and 4-hydroxy-tamoxifen, differentially affected mitochondrial superoxide production, with raloxifene acting as an agonist but 4-hydroxy-tamoxifen acting as an estrogen antagonist. Changes in superoxide by 17beta-estradiol could not be explained by changes in manganese superoxide dismutase. Instead, ERalpha-mediated decreases in mitochondrial ROS may depend on the concomitant increase in mitochondrial cytochrome c, previously shown to act as an antioxidant. Mitochondrial protective effects of estrogen in cerebral endothelium may contribute to sex differences in the occurrence of stroke and other age-related neurodegenerative diseases.
Figures
Similar articles
-
Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes.Mol Immunol. 2013 Dec;56(4):328-39. doi: 10.1016/j.molimm.2013.05.226. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911387
-
Estrogen suppresses brain mitochondrial oxidative stress in female and male rats.Brain Res. 2007 Oct 24;1176:71-81. doi: 10.1016/j.brainres.2007.08.036. Epub 2007 Aug 24. Brain Res. 2007. PMID: 17889838 Free PMC article.
-
17-β estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-β.Invest Ophthalmol Vis Sci. 2010 Oct;51(10):5278-87. doi: 10.1167/iovs.10-5316. Epub 2010 May 12. Invest Ophthalmol Vis Sci. 2010. PMID: 20463317
-
Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol.Ann N Y Acad Sci. 2005 Jun;1052:136-44. doi: 10.1196/annals.1347.009. Ann N Y Acad Sci. 2005. PMID: 16024756 Review.
-
Mechanisms of cerebrovascular protection: oestrogen, inflammation and mitochondria.Acta Physiol (Oxf). 2011 Sep;203(1):149-54. doi: 10.1111/j.1748-1716.2010.02184.x. Epub 2010 Oct 11. Acta Physiol (Oxf). 2011. PMID: 20825371 Free PMC article. Review.
Cited by
-
Endogenous ovarian hormones affect mitochondrial efficiency in cerebral endothelium via distinct regulation of PGC-1 isoforms.J Cereb Blood Flow Metab. 2013 Jan;33(1):122-8. doi: 10.1038/jcbfm.2012.159. Epub 2012 Oct 24. J Cereb Blood Flow Metab. 2013. PMID: 23093066 Free PMC article.
-
Age-related sex differences in the expression of important disease-linked mitochondrial proteins in mice.Biol Sex Differ. 2019 Dec 5;10(1):56. doi: 10.1186/s13293-019-0267-1. Biol Sex Differ. 2019. PMID: 31806023 Free PMC article.
-
Evaluation of Energy Balance on Human Telomerase Reverse Transcriptase (hTERT) Alternative Splicing by Semi-quantitative RT-PCR in Human Umbilical Vein Endothelial Cells.Adv Biomed Res. 2017 Apr 17;6:43. doi: 10.4103/2277-9175.204591. eCollection 2017. Adv Biomed Res. 2017. PMID: 28503498 Free PMC article.
-
Role of Sex Hormones on Brain Mitochondrial Function, with Special Reference to Aging and Neurodegenerative Diseases.Front Aging Neurosci. 2017 Dec 7;9:406. doi: 10.3389/fnagi.2017.00406. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29270123 Free PMC article. Review.
-
Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization.Endocrinology. 2012 Nov;153(11):5373-83. doi: 10.1210/en.2012-1458. Epub 2012 Aug 23. Endocrinology. 2012. PMID: 22919059 Free PMC article.
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. - PubMed
-
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas. 2000;34(Suppl 2):S35–52. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
-
- Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: a new paradigm for vascular protection? Mol Interv. 2006;6:26–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
