Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers
- PMID: 18350374
- PMCID: PMC2673943
- DOI: 10.1007/s12192-008-0031-7
Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers
Abstract
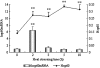
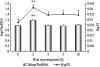
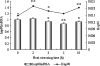
The objective of this study was to investigate the kinetics of Hsp60, Hsp70, Hsp90 protein, and messenger RNA (mRNA) expression levels and to correlate these heat shock protein (Hsp) levels with tissue damage resulting from exposure to high temperatures for varying amounts of time. One hundred broilers were heat-stressed for 0, 2, 3, 5, and 10 h, respectively, by rapidly increasing the ambient temperature from 22 +/- 1 degrees C to 37 +/- 1 degrees C. Obvious elevations of plasma creatine kinase indicate damage to myocardial cells after heat stress. Hsp70 and Hsp90, and their corresponding mRNAs in the heart tissue of heat-stressed broilers, elevated significantly after 2 h of heat exposure and decreased quickly with continued heat stress. However, the levels of hsp60 mRNA in the heart of heat-stressed broilers increased sharply (P < 0.01) at 2 h of heat stress but then decreased quickly after 3 h, while the level of Hsp60 protein in the heart increased (P < 0.01) at 2 h of heat stress and maintained a high level throughout heat exposure. The results indicate that the elevation of the three Hsps, especially Hsp60 in heart, may be important markers at the beginning of heat stress and act as protective proteins in adverse environments. The reduction of Hsp signals in the cytoplasm of myocardial cells implies that myocardial cell lesions may have an adverse impact on the function of Hsps during heat stress. Meanwhile, the localization of Hsp70 in blood vessels of broiler hearts suggests another possible mechanism for protection of the heart after heat exposure.
Figures

Similar articles
-
Expression and distribution of heat shock proteins in the heart of transported pigs.Cell Stress Chaperones. 2008 Dec;13(4):459-66. doi: 10.1007/s12192-008-0042-4. Epub 2008 May 9. Cell Stress Chaperones. 2008. PMID: 18465207 Free PMC article.
-
Gene expression of heat shoc kproteins/factors (HSP60, HSP70, HSP90, HSF-1, HSF-3) and antioxidant enzyme activities in heat stressed broilers treated with vitamin C.Pol J Vet Sci. 2019 Sep;22(3):565-572. doi: 10.24425/pjvs.2019.129965. Pol J Vet Sci. 2019. PMID: 31560472
-
Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature.Res Vet Sci. 2009 Jun;86(3):533-8. doi: 10.1016/j.rvsc.2008.09.002. Epub 2008 Oct 31. Res Vet Sci. 2009. PMID: 18951595
-
Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production.Poult Sci. 2018 Aug 1;97(8):2638-2646. doi: 10.3382/ps/pey146. Poult Sci. 2018. PMID: 29750253
-
Review: The role of heat shock proteins in chicken: Insights into stress adaptation and health.Res Vet Sci. 2023 Dec;165:105057. doi: 10.1016/j.rvsc.2023.105057. Epub 2023 Oct 16. Res Vet Sci. 2023. PMID: 37864906 Review.
Cited by
-
Effects of Macleaya cordata Extract on Blood Biochemical Indices and Intestinal Flora in Heat-Stressed Mice.Animals (Basel). 2022 Sep 28;12(19):2589. doi: 10.3390/ani12192589. Animals (Basel). 2022. PMID: 36230331 Free PMC article.
-
Localization and expression of Hsp27 and αB-crystallin in rat primary myocardial cells during heat stress in vitro.PLoS One. 2013 Jul 19;8(7):e69066. doi: 10.1371/journal.pone.0069066. Print 2013. PLoS One. 2013. PMID: 23894407 Free PMC article.
-
The association of SNPs in Hsp90β gene 5' flanking region with thermo tolerance traits and tissue mRNA expression in two chicken breeds.Mol Biol Rep. 2013 Sep;40(9):5295-306. doi: 10.1007/s11033-013-2630-3. Epub 2013 Jun 21. Mol Biol Rep. 2013. PMID: 23793829
-
Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease.Circulation. 2010 Oct 26;122(17):1740-51. doi: 10.1161/CIRCULATIONAHA.110.942250. Circulation. 2010. PMID: 20975010 Free PMC article. Review. No abstract available.
-
Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities.Cell Stress Chaperones. 2014 Nov;19(6):895-901. doi: 10.1007/s12192-014-0514-7. Epub 2014 May 6. Cell Stress Chaperones. 2014. PMID: 24796798 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9686751', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9686751/'}]}
- Benjamin IJ, McMillan DR (1998) Stress heat shock proteins molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0092-8674(00)80806-5', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0092-8674(00)80806-5'}, {'type': 'PubMed', 'value': '10786831', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10786831/'}]}
- Bukau B, Deuerling E, Pfund C, Craig EA (2000) Getting newly synthesized proteins into shape. Cell 101(2):119–122 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10653585', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10653585/'}]}
- Cechetto JD, Soltys BJ, Gupta RS (2000) Localization of mitochondrial 60-kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J Histochem Cytochem 48:45–56 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.3109/10409238509085135', 'is_inner': False, 'url': 'https://doi.org/10.3109/10409238509085135'}, {'type': 'PubMed', 'value': '2412760', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2412760/'}]}
- Craig EA (1985) The heat shock response. CRC Crit Rev Biochem 18:239–280 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0092-8674(94)90416-2', 'is_inner': False, 'url': 'https://doi.org/10.1016/0092-8674(94)90416-2'}, {'type': 'PubMed', 'value': '7914834', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7914834/'}]}
- Craig EA, Weissman JS, Horwich AL (1994) Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell 78:365–372 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

