Walleye dermal sarcoma virus Orf B functions through receptor for activated C kinase (RACK1) and protein kinase C
- PMID: 18343476
- PMCID: PMC2453751
- DOI: 10.1016/j.virol.2008.01.034
Walleye dermal sarcoma virus Orf B functions through receptor for activated C kinase (RACK1) and protein kinase C
Abstract
Walleye dermal sarcoma virus is a complex retrovirus that is associated with walleye dermal sarcomas that are seasonal in nature. Fall developing tumors contain low levels of spliced accessory gene transcripts A and B, suggesting a role for the encoded proteins, Orf A and Orf B, in oncogenesis. In explanted tumor cells the 35 kDa Orf B accessory protein is localized to the cell periphery in structures similar to focal adhesions and along actin stress fibers. Similar localization was observed in mammalian cells. The cellular protein, receptor for activated C kinase 1 (RACK1), bound Orf B in yeast two-hybrid assays and in cell culture. Sequence analysis of walleye RACK1 demonstrated high conservation to other known RACK1 sequences. RACK1 binds to activated protein kinase C (PKC). Orf B associates with PKCalpha, which is constitutively activated and localized at the membrane. Activated PKC promoted cell survival, proliferation, and increased cell viability in Orf B-expressing cells.
Figures
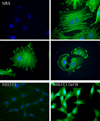


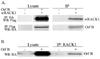
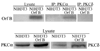


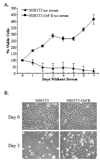
Similar articles
-
RACK1, a versatile hub in cancer.Oncogene. 2015 Apr 9;34(15):1890-8. doi: 10.1038/onc.2014.127. Epub 2014 Jun 2. Oncogene. 2015. PMID: 24882575 Review.
-
Walleye dermal sarcoma virus Orf C is targeted to the mitochondria.J Gen Virol. 2003 Feb;84(Pt 2):375-381. doi: 10.1099/vir.0.18570-0. J Gen Virol. 2003. PMID: 12560570
-
Interaction with receptor for activated C-kinase 1 (RACK1) sensitizes the phosphodiesterase PDE4D5 towards hydrolysis of cAMP and activation by protein kinase C.Biochem J. 2010 Nov 15;432(1):207-16. doi: 10.1042/BJ20101010. Biochem J. 2010. PMID: 20819076 Free PMC article.
-
Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins.Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):839-43. doi: 10.1073/pnas.91.3.839. Proc Natl Acad Sci U S A. 1994. PMID: 8302854 Free PMC article.
-
The cyclic AMP phosphodiesterase 4D5 (PDE4D5)/receptor for activated C-kinase 1 (RACK1) signalling complex as a sensor of the extracellular nano-environment.Cell Signal. 2017 Jul;35:282-289. doi: 10.1016/j.cellsig.2017.01.013. Epub 2017 Jan 6. Cell Signal. 2017. PMID: 28069443 Review.
Cited by
-
RACK1 is indispensable for porcine reproductive and respiratory syndrome virus replication and NF-κB activation in Marc-145 cells.Sci Rep. 2018 Feb 14;8(1):2985. doi: 10.1038/s41598-018-21460-4. Sci Rep. 2018. PMID: 29445214 Free PMC article.
-
RACK1, a versatile hub in cancer.Oncogene. 2015 Apr 9;34(15):1890-8. doi: 10.1038/onc.2014.127. Epub 2014 Jun 2. Oncogene. 2015. PMID: 24882575 Review.
-
The association of receptor of activated protein kinase C 1(RACK1) with infectious bursal disease virus viral protein VP5 and voltage-dependent anion channel 2 (VDAC2) inhibits apoptosis and enhances viral replication.J Biol Chem. 2015 Mar 27;290(13):8500-10. doi: 10.1074/jbc.M114.585687. Epub 2015 Jan 12. J Biol Chem. 2015. PMID: 25583988 Free PMC article.
-
Walleye dermal sarcoma virus: molecular biology and oncogenesis.Viruses. 2010 Sep;2(9):1984-1999. doi: 10.3390/v2091984. Epub 2010 Sep 22. Viruses. 2010. PMID: 21994717 Free PMC article.
-
Walleye dermal sarcoma virus: expression of a full-length clone or the rv-cyclin (orf a) gene is cytopathic to the host and human tumor cells.Mol Biol Rep. 2013 Feb;40(2):1451-61. doi: 10.1007/s11033-012-2188-5. Epub 2012 Oct 26. Mol Biol Rep. 2013. PMID: 23100064
References
-
- Bowser PR, Martineau D, Wooster GA. Effects of water temperature on experimental transmission of dermal sarcoma in fingerling walleyes (Stizostedion vitreum) Journal of Aquatic Animal Health. 1990;2:157–161. - PubMed
-
- Bowser PR, Wolfe MJ, Forney JL, Wooster GA. Seasonal prevalence of skin tumors from walleye (Stizostedion vitreum) from Oneida Lake, New York. Journal of Wildlife Diseases. 1988;24:292–298. - PubMed
-
- Bowser PR, Wooster GA. Regression of dermal sarcoma in adult walleyes (Stizostedion vitreum) Journal of Aquatic Animal Health. 1991;3:147–150.
-
- Bowser PR, Wooster GA, Quackenbush SL, Casey RN, Casey JW. Comparison of fall and spring tumors as inocula for experimental transmission of walleye dermal sarcoma. Journal of Aquatic Animal Health. 1996;8:78–81.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

