Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus
- PMID: 18334634
- PMCID: PMC2393785
- DOI: 10.1073/pnas.0800422105
Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus
Abstract
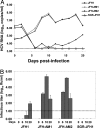
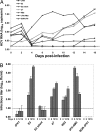
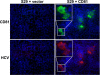
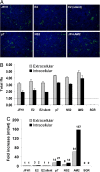
The JFH1 strain of hepatitis C virus (HCV) is unique among HCV isolates, in that the wild-type virus can traverse the entire replication cycle in cultured cells. However, without adaptive mutations, only low levels of infectious virus are produced. In the present study, the effects of five mutations that were selected during serial passage in Huh-7.5 cells were studied. Recombinant genomes containing all five mutations produced 3-4 logs more infectious virions than did wild type. Neither a coding mutation in NS5A nor a silent mutation in E2 was adaptive, whereas coding mutations in E2, p7, and NS2 all increased virus production. A single-cycle replication assay in CD81-deficient cells was developed to study more precisely the effect of the adaptive mutations. The E2 mutation had minimal effect on the amount of infectious virus released but probably enhanced entry into cells. In contrast, both the p7 and NS2 mutations independently increased the amount of virus released.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Efficient production of infectious hepatitis C virus with adaptive mutations in cultured hepatoma cells.J Gen Virol. 2009 Jul;90(Pt 7):1681-1691. doi: 10.1099/vir.0.010983-0. Epub 2009 Mar 12. J Gen Virol. 2009. PMID: 19282429
-
Cell culture-adaptive mutations in hepatitis C virus promote viral production by enhancing viral replication and release.World J Gastroenterol. 2018 Mar 28;24(12):1299-1311. doi: 10.3748/wjg.v24.i12.1299. World J Gastroenterol. 2018. PMID: 29599605 Free PMC article.
-
Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses.Gastroenterology. 2007 Nov;133(5):1614-26. doi: 10.1053/j.gastro.2007.08.005. Epub 2007 Aug 3. Gastroenterology. 2007. PMID: 17983807
-
Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus.J Virol. 2007 Jan;81(2):629-38. doi: 10.1128/JVI.01890-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079282 Free PMC article.
-
Amino Acid Mutations in the NS4A Region of Hepatitis C Virus Contribute to Viral Replication and Infectious Virus Production.J Virol. 2017 Jan 31;91(4):e02124-16. doi: 10.1128/JVI.02124-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928005 Free PMC article.
Cited by
-
Cell culture-adaptive mutations promote viral protein-protein interactions and morphogenesis of infectious hepatitis C virus.J Virol. 2012 Sep;86(17):8987-97. doi: 10.1128/JVI.00004-12. Epub 2012 Jun 6. J Virol. 2012. PMID: 22674987 Free PMC article.
-
A Recombinant Hepatitis C Virus Genotype 1a E1/E2 Envelope Glycoprotein Vaccine Elicits Antibodies That Differentially Neutralize Closely Related 2a Strains through Interactions of the N-Terminal Hypervariable Region 1 of E2 with Scavenger Receptor B1.J Virol. 2019 Oct 29;93(22):e00810-19. doi: 10.1128/JVI.00810-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462563 Free PMC article.
-
Generation of a cell culture-adapted hepatitis C virus with longer half life at physiological temperature.PLoS One. 2011;6(8):e22808. doi: 10.1371/journal.pone.0022808. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21829654 Free PMC article.
-
The association of CD81 with tetraspanin-enriched microdomains is not essential for Hepatitis C virus entry.BMC Microbiol. 2009 May 28;9:111. doi: 10.1186/1471-2180-9-111. BMC Microbiol. 2009. PMID: 19476617 Free PMC article.
-
Identification of a novel drug lead that inhibits HCV infection and cell-to-cell transmission by targeting the HCV E2 glycoprotein.PLoS One. 2014 Oct 30;9(10):e111333. doi: 10.1371/journal.pone.0111333. eCollection 2014. PLoS One. 2014. PMID: 25357246 Free PMC article.
References
-
- Bowen DG, Walker CM. The origin of quasispecies: cause or consequence of chronic hepatitis C viral infection? J Hepatol. 2005;42:408–417. - PubMed
-
- Lindenbach BD, Rice CM. In: Fields Virology. 5th Ed. Knipe DM, Howley PM, editors. Vol 1. Philadelphia: Lippincott–Raven; 2007. pp. 1101–1152.
-
- Kato T, et al. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J Med Virol. 2001;64:334–339. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

