Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells
- PMID: 18332220
- PMCID: PMC2265407
- DOI: 10.1083/jcb.200708043
Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells
Abstract
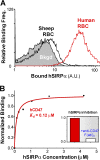
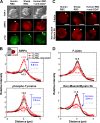
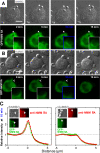
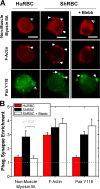
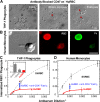
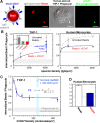
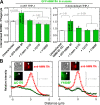
Phagocytosis of foreign cells or particles by macrophages is a rapid process that is inefficient when faced with "self" cells that display CD47-although signaling mechanisms in self-recognition have remained largely unknown. With human macrophages, we show the phagocytic synapse at cell contacts involves a basal level of actin-driven phagocytosis that, in the absence of species-specific CD47 signaling, is made more efficient by phospho-activated myosin. We use "foreign" sheep red blood cells (RBCs) together with CD47-blocked, antibody-opsonized human RBCs in order to visualize synaptic accumulation of phosphotyrosine, paxillin, F-actin, and the major motor isoform, nonmuscle myosin-IIA. When CD47 is functional, the macrophage counter-receptor and phosphatase-activator SIRPalpha localizes to the synapse, suppressing accumulation of phosphotyrosine and myosin without affecting F-actin. On both RBCs and microbeads, human CD47 potently inhibits phagocytosis as does direct inhibition of myosin. CD47-SIRPalpha interaction initiates a dephosphorylation cascade directed in part at phosphotyrosine in myosin. A point mutation turns off this motor's contribution to phagocytosis, suggesting that self-recognition inhibits contractile engulfment.
Figures



Similar articles
-
Cell rigidity and shape override CD47's "self"-signaling in phagocytosis by hyperactivating myosin-II.Blood. 2015 Jan 15;125(3):542-52. doi: 10.1182/blood-2014-06-585299. Epub 2014 Nov 19. Blood. 2015. PMID: 25411427 Free PMC article.
-
Macrophages show higher levels of engulfment after disruption of cis interactions between CD47 and the checkpoint receptor SIRPα.J Cell Sci. 2020 Mar 6;133(5):jcs237800. doi: 10.1242/jcs.237800. J Cell Sci. 2020. PMID: 31964705 Free PMC article.
-
Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina.Immunity. 2022 Dec 13;55(12):2318-2335.e7. doi: 10.1016/j.immuni.2022.10.018. Epub 2022 Nov 14. Immunity. 2022. PMID: 36379210 Free PMC article.
-
Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway.Trends Cell Biol. 2009 Feb;19(2):72-80. doi: 10.1016/j.tcb.2008.12.001. Epub 2009 Jan 12. Trends Cell Biol. 2009. PMID: 19144521 Review.
-
CD47-SIRPα Axis as a Biomarker and Therapeutic Target in Cancer: Current Perspectives and Future Challenges in Nonsmall Cell Lung Cancer.J Immunol Res. 2020 Sep 19;2020:9435030. doi: 10.1155/2020/9435030. eCollection 2020. J Immunol Res. 2020. PMID: 33015199 Free PMC article. Review.
Cited by
-
Harnessing and Enhancing Macrophage Phagocytosis for Cancer Therapy.Front Immunol. 2021 Mar 10;12:635173. doi: 10.3389/fimmu.2021.635173. eCollection 2021. Front Immunol. 2021. PMID: 33790906 Free PMC article. Review.
-
Phagocytosis Checkpoints in Glioblastoma: CD47 and Beyond.Curr Issues Mol Biol. 2024 Jul 23;46(8):7795-7811. doi: 10.3390/cimb46080462. Curr Issues Mol Biol. 2024. PMID: 39194679 Free PMC article. Review.
-
Programmed cell removal: a new obstacle in the road to developing cancer.Nat Rev Cancer. 2011 Dec 8;12(1):58-67. doi: 10.1038/nrc3171. Nat Rev Cancer. 2011. PMID: 22158022 Review.
-
Silencing of CD47 and SIRPα by Polypurine reverse Hoogsteen hairpins to promote MCF-7 breast cancer cells death by PMA-differentiated THP-1 cells.BMC Immunol. 2016 Sep 26;17(1):32. doi: 10.1186/s12865-016-0170-z. BMC Immunol. 2016. PMID: 27671753 Free PMC article.
-
The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications.Curr Opin Immunol. 2012 Apr;24(2):225-32. doi: 10.1016/j.coi.2012.01.010. Epub 2012 Feb 4. Curr Opin Immunol. 2012. PMID: 22310103 Free PMC article. Review.
References
-
- Aderem, A., and D.M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623. - PubMed
-
- Arndt, P.A., and G. Garratty. 2004. Rh(null) red blood cells with reduced CD47 do not show increased interactions with peripheral blood monocytes. Br. J. Haematol. 125:412–414. - PubMed
-
- Baba, T., N. Fusaki, N. Shinya, A. Iwamatsu, and N. Hozumi. 2003. Myosin is an in vivo substrate of the protein tyrosine phosphatase (SHP-1) after mIgM cross-linking. Biochem. Biophys. Res. Commun. 304:67–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

