Long-term retention on treatment with lumiracoxib 100 mg once or twice daily compared with celecoxib 200 mg once daily: a randomised controlled trial in patients with osteoarthritis
- PMID: 18328090
- PMCID: PMC2322990
- DOI: 10.1186/1471-2474-9-32
Long-term retention on treatment with lumiracoxib 100 mg once or twice daily compared with celecoxib 200 mg once daily: a randomised controlled trial in patients with osteoarthritis
Abstract
Background: The efficacy, safety and tolerability of lumiracoxib, a novel selective cyclooxygenase-2 (COX-2) inhibitor, has been demonstrated in previous studies of patients with osteoarthritis (OA). As it is important to establish the long-term safety and efficacy of treatments for a chronic disease such as OA, the present study compared the effects of lumiracoxib at doses of 100 mg once daily (o.d.) and 100 mg twice daily (b.i.d.) with those of celecoxib 200 mg o.d. on retention on treatment over 1 year.
Methods: In this 52-week, multicentre, randomised, double-blind, parallel-group study, male and female patients (aged at least 40 years) with symptomatic primary OA of the hip, knee, hand or spine were randomised (1:2:1) to lumiracoxib 100 mg o.d. (n = 755), lumiracoxib 100 mg b.i.d. (n = 1,519) or celecoxib 200 mg o.d. (n = 758). The primary objective of the study was to demonstrate non-inferiority of lumiracoxib at either dose compared with celecoxib 200 mg o.d. with respect to the 1-year retention on treatment rate. Secondary outcome variables included OA pain in the target joint, patient's and physician's global assessments of disease activity, Short Arthritis assessment Scale (SAS) total score, rescue medication use, and safety and tolerability.
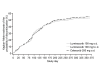
Results: Retention rates at 1 year were similar for the lumiracoxib 100 mg o.d., lumiracoxib 100 mg b.i.d. and celecoxib 200 mg o.d. groups (46.9% vs 47.5% vs 45.3%, respectively). It was demonstrated that retention on treatment with lumiracoxib at either dose was non-inferior to celecoxib 200 mg o.d. Similarly, Kaplan-Meier curves for the probability of premature discontinuation from the study for any reason were similar across the treatment groups. All three treatments generally yielded comparable results for the secondary efficacy variables and all treatments were well tolerated.
Conclusion: Long-term treatment with lumiracoxib 100 mg o.d., the recommended dose for OA, was as effective and well tolerated as celecoxib 200 mg o.d. in patients with OA.
Trial registration: clinicaltrials.gov NCT00145301.
Figures

Similar articles
-
First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215].Arthritis Res Ther. 2006;8(2):R35. doi: 10.1186/ar1854. Epub 2006 Jan 16. Arthritis Res Ther. 2006. PMID: 16469112 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of lumiracoxib 100 mg once daily in knee osteoarthritis: a 13-week, randomized, double-blind study vs. placebo and celecoxib.Curr Med Res Opin. 2005 Apr;21(4):517-26. doi: 10.1185/030079905x38196. Curr Med Res Opin. 2005. PMID: 15899100 Clinical Trial.
-
Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a prospective randomized 13-week study versus placebo and celecoxib.Clin Rheumatol. 2006 Feb;25(1):42-53. doi: 10.1007/s10067-005-1126-5. Epub 2005 Aug 13. Clin Rheumatol. 2006. PMID: 16132165 Clinical Trial.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2008 Apr;12(11):1-278, iii. doi: 10.3310/hta12110. Health Technol Assess. 2008. PMID: 18405470 Review.
-
Clinical pharmacology of lumiracoxib, a second-generation cyclooxygenase 2 selective inhibitor.Expert Opin Investig Drugs. 2005 Apr;14(4):521-33. doi: 10.1517/13543784.14.4.521. Expert Opin Investig Drugs. 2005. PMID: 15882125 Review.
Cited by
-
A 6-week, multicentre, randomised, double-blind, double-dummy, active-controlled, clinical safety study of lumiracoxib and rofecoxib in osteoarthritis patients.BMC Musculoskelet Disord. 2008 Sep 8;9:118. doi: 10.1186/1471-2474-9-118. BMC Musculoskelet Disord. 2008. PMID: 18778469 Free PMC article. Clinical Trial.
-
Systematic review and network meta-analysis on the efficacy and safety of parmacotherapy for hand osteoarthritis.PLoS One. 2024 May 9;19(5):e0298774. doi: 10.1371/journal.pone.0298774. eCollection 2024. PLoS One. 2024. PMID: 38722915 Free PMC article.
-
Systematic Review of Non-surgical Therapies for Osteoarthritis of the Hand: An Update.Eur J Rheumatol. 2023 Feb 6;11(Suppl 1):S53-67. doi: 10.5152/eurjrheum.2023.21197. Online ahead of print. Eur J Rheumatol. 2023. PMID: 36744772 Free PMC article.
-
Preemptive versus postoperative lumiracoxib for analgesia in ambulatory arthroscopic knee surgery.J Pain Res. 2008 Nov 1;1:27-34. doi: 10.2147/jpr.s3928. J Pain Res. 2008. PMID: 21197285 Free PMC article.
-
Paracetamol and the placebo effect in osteoarthritis trials: a missing link?Pain Res Treat. 2011;2011:696791. doi: 10.1155/2011/696791. Epub 2011 Jun 6. Pain Res Treat. 2011. PMID: 22110930 Free PMC article.
References
-
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martín-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee forInternational Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. - DOI - PMC - PubMed
-
- Murray CJL, Lopez AD, Eds . The global burden of disease. A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge, MA; Harvard School of Public Health on behalf of the World Health Organization and The World Bank; 1996.
-
- American College of Rheumatology Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

