Epigenetic regulation of BMP7 in the regenerative response to ischemia
- PMID: 18322163
- PMCID: PMC2440290
- DOI: 10.1681/ASN.2007091040
Epigenetic regulation of BMP7 in the regenerative response to ischemia
Abstract
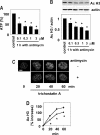
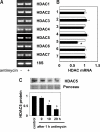
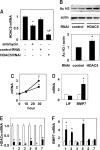
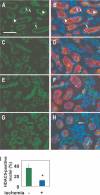
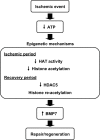
Kidneys damaged by ischemia have the potential to regenerate through a mechanism involving intrarenal induction of protective factors, including bone morphogenetic protein-7 (BMP7). Epigenetic changes, such as alterations in histone modifications, have also been shown to play a role in various pathologic conditions, but their involvement in ischemic injury and regeneration remains unknown. This study investigated whether changes in histone acetylation, regulated by histone acetyltransferase and histone deacetylase (HDAC), are induced by renal ischemia and involved in the regenerative response. Ischemia/reperfusion of the mouse kidney induced a transient decrease in histone acetylation in proximal tubular cells, likely as a result of a decrease in histone acetyltransferase activity as suggested by experiments with energy-depleted renal epithelial cells in culture. During recovery after transient energy depletion in epithelial cells, the HDAC isozyme HDAC5 was selectively downregulated in parallel with the return of acetylated histone. Knockdown of HDAC5 by RNAi significantly increased histone acetylation and BMP7 expression. BMP7 induction and HDAC5 downregulation in the recovery phase were also observed in proximal tubular cells in vivo after transient ischemia. These data indicate that ischemia induces dynamic epigenetic changes involving HDAC5 downregulation, which contributes to histone re-acetylation and BMP7 induction in the recovery phase. This highlights HDAC5 as a modulator of the regenerative response after ischemia and suggests HDAC5 inhibition may be a therapeutic strategy to enhance BMP7 expression.
Figures



Similar articles
-
Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy.J Am Soc Nephrol. 2001 Nov;12(11):2392-2399. doi: 10.1681/ASN.V12112392. J Am Soc Nephrol. 2001. PMID: 11675415
-
METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease.Cell Death Dis. 2021 Jan 4;12(1):32. doi: 10.1038/s41419-020-03312-0. Cell Death Dis. 2021. PMID: 33414476 Free PMC article.
-
Nucleocytoplasmic export of HDAC5 and SIRT2 downregulation: two epigenetic mechanisms by which antidepressants enhance synaptic plasticity markers.Psychopharmacology (Berl). 2018 Oct;235(10):2831-2846. doi: 10.1007/s00213-018-4975-8. Epub 2018 Aug 8. Psychopharmacology (Berl). 2018. PMID: 30091005
-
Epigenetic regulation of airway inflammation.Curr Opin Immunol. 2007 Dec;19(6):694-700. doi: 10.1016/j.coi.2007.07.016. Epub 2007 Aug 27. Curr Opin Immunol. 2007. PMID: 17720468 Review.
-
Histone Acetylation Enzymes Coordinate Metabolism and Gene Expression.Trends Plant Sci. 2015 Oct;20(10):614-621. doi: 10.1016/j.tplants.2015.07.005. Trends Plant Sci. 2015. PMID: 26440431 Review.
Cited by
-
Regulation of embryonic kidney branching morphogenesis and glomerular development by KISS1 receptor (Gpr54) through NFAT2- and Sp1-mediated Bmp7 expression.J Biol Chem. 2010 Jun 4;285(23):17811-20. doi: 10.1074/jbc.M110.130740. Epub 2010 Apr 7. J Biol Chem. 2010. PMID: 20375015 Free PMC article.
-
Histone acetylation and DNA methylation in ischemia/reperfusion injury.Clin Sci (Lond). 2019 Feb 25;133(4):597-609. doi: 10.1042/CS20180465. Print 2019 Feb 28. Clin Sci (Lond). 2019. PMID: 30804072 Free PMC article. Review.
-
Zebrafish kidney development: basic science to translational research.Birth Defects Res C Embryo Today. 2011 Jun;93(2):141-56. doi: 10.1002/bdrc.20209. Birth Defects Res C Embryo Today. 2011. PMID: 21671354 Free PMC article. Review.
-
The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction.Am J Physiol Renal Physiol. 2016 Jul 1;311(1):F145-61. doi: 10.1152/ajprenal.00164.2016. Epub 2016 May 18. Am J Physiol Renal Physiol. 2016. PMID: 27194714 Free PMC article. Review.
-
Class IIa histone deacetylase inhibition ameliorates acute kidney injury by suppressing renal tubular cell apoptosis and enhancing autophagy and proliferation.Front Pharmacol. 2022 Jul 22;13:946192. doi: 10.3389/fphar.2022.946192. eCollection 2022. Front Pharmacol. 2022. PMID: 35935816 Free PMC article.
References
-
- Bolden JE, Peart MJ, Johnstone RW: Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784, 2006 - PubMed
-
- Bernstein BE, Meissner A, Lander ES: The mammalian epigenome. Cell 128: 669–681, 2007 - PubMed
-
- Reik W: Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447: 425–432, 2007 - PubMed
-
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK: Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435: 1262–1266, 2005 - PubMed
-
- Backs J, Olson EN: Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

