A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis
- PMID: 18316367
- PMCID: PMC2376231
- DOI: 10.1074/jbc.M800564200
A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis
Abstract
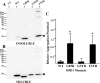
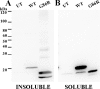
One of the mechanisms by which mutations in superoxide dismutase 1 (SOD1) cause familial amyotrophic lateral sclerosis (fALS) is proposed to involve the accumulation of detergent-insoluble, disulfide-cross-linked, mutant protein. Recent studies have implicated cysteine residues at positions 6 and 111 as critical in mediating disulfide cross-linking and promoting aggregation. In the present study, we used a panel of experimental and disease-linked mutations at cysteine residues of SOD1 (positions 6, 57, 111, and 146) in cell culture assays for aggregation to demonstrate that extensive disulfide cross-linking is not required for the formation of mutant SOD1 aggregates. Experimental mutants possessing only a single cysteine residue or lacking cysteine entirely were found to retain high potential to aggregate. Furthermore we demonstrate that aggregate structures in symptomatic SOD1-G93A mice can be dissociated such that they no longer sediment upon ultracentrifugation (i.e. appear soluble) under relatively mild conditions that leave disulfide bonds intact. Similar to other recent work, we found that cysteines 6 and 111, particularly the latter, play interesting roles in modulating the aggregation of human SOD1. However, we did not find that extensive disulfide cross-linking via these residues, or any other cysteine, is critical to aggregate structure. Instead we suggest that these residues participate in other features of the protein that, in some manner, modulate aggregation.
Figures
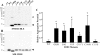

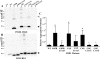


Similar articles
-
Role of disulfide cross-linking of mutant SOD1 in the formation of inclusion-body-like structures.PLoS One. 2012;7(10):e47838. doi: 10.1371/journal.pone.0047838. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118898 Free PMC article.
-
Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1.J Biol Chem. 2007 Sep 21;282(38):28087-95. doi: 10.1074/jbc.M704465200. Epub 2007 Jul 31. J Biol Chem. 2007. PMID: 17666395
-
Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS.Proc Natl Acad Sci U S A. 2009 May 12;106(19):7774-9. doi: 10.1073/pnas.0902505106. Epub 2009 Apr 30. Proc Natl Acad Sci U S A. 2009. PMID: 19416874 Free PMC article.
-
Immature copper-zinc superoxide dismutase and familial amyotrophic lateral sclerosis.Exp Biol Med (Maywood). 2009 Oct;234(10):1140-54. doi: 10.3181/0903-MR-104. Epub 2009 Jul 13. Exp Biol Med (Maywood). 2009. PMID: 19596823 Free PMC article. Review.
-
SOD1 aggregation and ALS: role of metallation states and disulfide status.Curr Top Med Chem. 2012;12(22):2560-72. doi: 10.2174/1568026611212220010. Curr Top Med Chem. 2012. PMID: 23339308 Review.
Cited by
-
Relationship between mutant Cu/Zn superoxide dismutase 1 maturation and inclusion formation in cell models.J Neurochem. 2017 Jan;140(1):140-150. doi: 10.1111/jnc.13864. Epub 2016 Nov 25. J Neurochem. 2017. PMID: 27727458 Free PMC article.
-
Role of disulfide cross-linking of mutant SOD1 in the formation of inclusion-body-like structures.PLoS One. 2012;7(10):e47838. doi: 10.1371/journal.pone.0047838. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118898 Free PMC article.
-
Probing the free energy landscapes of ALS disease mutants of SOD1 by NMR spectroscopy.Proc Natl Acad Sci U S A. 2016 Nov 8;113(45):E6939-E6945. doi: 10.1073/pnas.1611418113. Epub 2016 Oct 24. Proc Natl Acad Sci U S A. 2016. PMID: 27791136 Free PMC article.
-
Oxidized SOD1 accelerates cellular senescence in neural stem cells.Stem Cell Res Ther. 2024 Feb 27;15(1):55. doi: 10.1186/s13287-024-03669-5. Stem Cell Res Ther. 2024. PMID: 38414053 Free PMC article.
-
A novel variant of human superoxide dismutase 1 harboring amyotrophic lateral sclerosis-associated and experimental mutations in metal-binding residues and free cysteines lacks toxicity in vivo.J Neurochem. 2012 May;121(3):475-85. doi: 10.1111/j.1471-4159.2012.07690.x. Epub 2012 Mar 20. J Neurochem. 2012. PMID: 22332887 Free PMC article.
References
-
- Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., Rahmani, Z., Krizus, A., McKenna-Yasek, D., Cayabyab, A., Gaston, S. M., Berger, R., Tanzi, R. E., Halperin, J. J., Herzfeldt, B., Van den Bergh, R., Hung, W.-Y., Bird, T., Deng, G., Mulder, D. W., Smyth, C., Laing, N. G., Soriano, E., Pericak-Vance, M. A., Haines, J., Rouleau, G. A., Gusella, J. S., Horvitz, H. R., and Brown, R. H., Jr. (1993) Nature 362 59–62 - PubMed
-
- Lyons, T. J., Gralla, E. B., and Valentine, J. S. (1999) Met. Ions Biol. Syst. 36 125–177 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

