The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway
- PMID: 18311165
- PMCID: PMC2597498
- DOI: 10.1038/nrmicro1855
The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway
Abstract
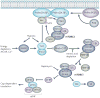
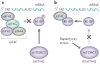
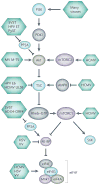
The successful replication of mammalian DNA viruses requires that they gain control of key cellular signalling pathways that affect broad aspects of cellular macromolecular synthesis, metabolism, growth and survival. The phosphatidylinositol 3'-kinase-Akt-mammalian target of rapamycin (PI3K-Akt-mTOR) pathway is one such pathway. Mammalian DNA viruses have evolved various mechanisms to activate this pathway to obtain the benefits of Akt activation, including the maintenance of translation through the activation of mTOR. In addition, viruses must overcome the inhibition of this pathway that results from the activation of cellular stress responses during viral infection. This Review will discuss the range of mechanisms that mammalian DNA viruses use to activate this pathway, as well as the multiple mechanisms these viruses have evolved to circumvent inhibitory stress signalling.
Figures

Similar articles
-
Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway.J Virol. 2007 Sep;81(18):10072-80. doi: 10.1128/JVI.00541-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609269 Free PMC article.
-
Differential Phosphatidylinositol-3-Kinase-Akt-mTOR Activation by Semliki Forest and Chikungunya Viruses Is Dependent on nsP3 and Connected to Replication Complex Internalization.J Virol. 2015 Nov;89(22):11420-37. doi: 10.1128/JVI.01579-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339054 Free PMC article.
-
[Research advances on the relationship of PI3-kinase/Akt/mTOR pathway and epigenetic modification].Yi Chuan. 2006 Dec;28(12):1585-90. doi: 10.1360/yc-006-1585. Yi Chuan. 2006. PMID: 17138547 Review. Chinese.
-
Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy.Cancer Res. 2005 Sep 15;65(18):8423-32. doi: 10.1158/0008-5472.CAN-05-0058. Cancer Res. 2005. PMID: 16166321
-
Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside.Curr Med Chem. 2007;14(19):2009-23. doi: 10.2174/092986707781368423. Curr Med Chem. 2007. PMID: 17691943 Review.
Cited by
-
MCV and Merkel cell carcinoma: a molecular success story.Curr Opin Virol. 2012 Aug;2(4):489-98. doi: 10.1016/j.coviro.2012.05.007. Epub 2012 Jun 17. Curr Opin Virol. 2012. PMID: 22710026 Free PMC article. Review.
-
Interactions of Hepatitis B Virus Infection with Nonalcoholic Fatty Liver Disease: Possible Mechanisms and Clinical Impact.Dig Dis Sci. 2015 Dec;60(12):3513-24. doi: 10.1007/s10620-015-3772-z. Epub 2015 Jun 26. Dig Dis Sci. 2015. PMID: 26112990 Review.
-
CEACAM1-Mediated Inhibition of Virus Production.Cell Rep. 2016 Jun 14;15(11):2331-9. doi: 10.1016/j.celrep.2016.05.036. Epub 2016 Jun 2. Cell Rep. 2016. PMID: 27264178 Free PMC article.
-
Viruses, microRNAs, and host interactions.Annu Rev Microbiol. 2010;64:123-41. doi: 10.1146/annurev.micro.112408.134243. Annu Rev Microbiol. 2010. PMID: 20477536 Free PMC article. Review.
-
The 5' leader of the mRNA encoding the marek's disease virus serotype 1 pp14 protein contains an intronic internal ribosome entry site with allosteric properties.J Virol. 2009 Dec;83(24):12769-78. doi: 10.1128/JVI.01010-09. Epub 2009 Sep 30. J Virol. 2009. PMID: 19793814 Free PMC article.
References
-
- Cooray S. The pivotal role of phosphatidylinositol-3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. - PubMed
-
An overview of the importance of the control of Akt to most viruses.
-
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. - PubMed
-
- Kaufman RJ, et al. The unfolded protein response in nutrient sensing and differentiation. Nature Rev Mol Cell Biol. 2002;3:411–421. - PubMed
-
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. - PubMed
-
- Wouters BG, et al. Control of the hypoxic response through regulation of mRNA translation. Sem Cell Dev Biol. 2005;16:487–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

