Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice
- PMID: 18308938
- PMCID: PMC2268794
- DOI: 10.1073/pnas.0711700105
Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice
Abstract
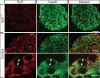
Vertebrates express at least 15 different synaptotagmins with the same domain structure but diverse localizations and tissue distributions. Synaptotagmin-1,-2, and -9 act as calcium sensors for the fast phrase of neurotransmitter release, and synaptotagmin-12 acts as a calcium-independent modulator of release. The exact functions of the remaining 11 synaptotagmins, however, have not been established. By analogy to the role of synaptotagmin-1, -2, and -9 in neurotransmission, these other synaptotagmins may serve as Ca(2+) transducers regulating other Ca(2+)-dependent membrane processes, such as insulin secretion in pancreatic beta-cells. Of these other synaptotagmins, synaptotagmin-7 is one of the most abundant and is present in pancreatic beta-cells. To determine whether synaptotagmin-7 regulates Ca(2+)-dependent insulin secretion, we analyzed synaptotagmin-7 null mutant mice for glucose tolerance and insulin release. Here, we show that synaptotagmin-7 is required for the maintenance of systemic glucose tolerance and glucose-stimulated insulin secretion. Mutant mice have normal insulin sensitivity, insulin production, islet architecture and ultrastructural organization, and metabolic and calcium responses but exhibit impaired glucose-induced insulin secretion, indicating a calcium-sensing defect during insulin-containing secretory granule exocytosis. Taken together, our findings show that synaptotagmin-7 functions as a positive regulator of insulin secretion and may serve as a calcium sensor controlling insulin secretion in pancreatic beta cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
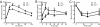

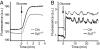
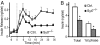
Similar articles
-
Neuronal calcium sensor synaptotagmin-9 is not involved in the regulation of glucose homeostasis or insulin secretion.PLoS One. 2010 Nov 9;5(11):e15414. doi: 10.1371/journal.pone.0015414. PLoS One. 2010. PMID: 21085706 Free PMC article.
-
Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from β-cells.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9996-10001. doi: 10.1073/pnas.1513004112. Epub 2015 Jul 27. Proc Natl Acad Sci U S A. 2015. PMID: 26216970 Free PMC article.
-
Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca2+ Sensitivity of Insulin Secretion Vesicles.Dev Cell. 2018 May 7;45(3):347-361.e5. doi: 10.1016/j.devcel.2018.03.013. Epub 2018 Apr 12. Dev Cell. 2018. PMID: 29656931 Free PMC article.
-
Synaptotagmins bind calcium to release insulin.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1279-86. doi: 10.1152/ajpendo.90568.2008. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713958 Review.
-
Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion.Biosci Rep. 2009 Aug;29(4):245-59. doi: 10.1042/BSR20090031. Biosci Rep. 2009. PMID: 19500075 Review.
Cited by
-
Autophagy is involved in adipogenic differentiation by repressesing proteasome-dependent PPARγ2 degradation.Am J Physiol Endocrinol Metab. 2013 Aug 15;305(4):E530-9. doi: 10.1152/ajpendo.00640.2012. Epub 2013 Jun 25. Am J Physiol Endocrinol Metab. 2013. PMID: 23800883 Free PMC article.
-
Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23.PLoS One. 2014 Nov 25;9(11):e114033. doi: 10.1371/journal.pone.0114033. eCollection 2014. PLoS One. 2014. PMID: 25422940 Free PMC article.
-
Synaptotagmin-1 and -7 Are Redundantly Essential for Maintaining the Capacity of the Readily-Releasable Pool of Synaptic Vesicles.PLoS Biol. 2015 Oct 5;13(10):e1002267. doi: 10.1371/journal.pbio.1002267. eCollection 2015 Oct. PLoS Biol. 2015. PMID: 26437117 Free PMC article.
-
Differential Membrane Binding Mechanics of Synaptotagmin Isoforms Observed in Atomic Detail.Biochemistry. 2017 Jan 10;56(1):281-293. doi: 10.1021/acs.biochem.6b00468. Epub 2016 Dec 20. Biochemistry. 2017. PMID: 27997124 Free PMC article.
-
High-speed imaging reveals the bimodal nature of dense core vesicle exocytosis.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2214897120. doi: 10.1073/pnas.2214897120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574702 Free PMC article.
References
-
- Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. - PubMed
-
- Ashcroft FM, Rorsman P. Molecular defects in insulin secretion in type-2 diabetes. Rev Endocr Metab Disord. 2004;5:135–142. - PubMed
-
- Henquin J.-C., et al. Signals and Pools Underlying Biphasic Insulin Secretion. Diabetes. 2002;51:S60–S67. - PubMed
-
- Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. - PubMed
-
- Kahn SE, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5824–5829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

