Enzymatically inactive U(S)3 protein kinase of Marek's disease virus (MDV) is capable of depolymerizing F-actin but results in accumulation of virions in perinuclear invaginations and reduced virus growth
- PMID: 18304599
- PMCID: PMC2430872
- DOI: 10.1016/j.virol.2008.01.026
Enzymatically inactive U(S)3 protein kinase of Marek's disease virus (MDV) is capable of depolymerizing F-actin but results in accumulation of virions in perinuclear invaginations and reduced virus growth
Abstract
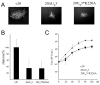
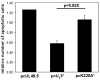
Marek's disease (MD) is a highly contagious, lymphoproliferative disease of chickens caused by the cell-associated MD virus (MDV), a member of the alphaherpesvirus subfamily. In a previous study we showed that the absence of the serine/threonine protein kinase (pU(S)3) encoded in the MDV unique-short region resulted in accumulation of primarily enveloped virions in the perinuclear space and significant impairment of virus growth in vitro. It was also shown that pU(S)3 is involved in actin stress fiber breakdown [Schumacher, D., Tischer, B. K., Trapp, S., and Osterrieder, N. (2005). Here, we constructed a recombinant virus to test the importance of pU(S)3 kinase activity for MDV replication and its functions in actin rearrangement. Disruption of the kinase active site was achieved by substituting a lysine at position 220 with an alanine (K220A). Titers of a kinase-negative MDV mutant, 20U(S)3()K220A, were reduced when compared to parental virus similar to those of the U(S)3 deletion mutant. We were also able to demonstrate complete absence of phosphorylation of MDV-specific phosphoprotein pp38 in cells infected with the kinase-deficient virus, indicating that pp38 phosphorylation depends entirely on the kinase activity of pU(S)3. Enzymatically inactive pU(S)3()K220A was, however, still capable of mediating breakdown of the actin cytoskeleton in transfection studies, and this activity was indistinguishable from that of wild-type pU(S)3(). Furthermore, we demonstrated that pU(S)3 possesses anti-apoptotic activity, which is dependent on its kinase activity. Taken together, our results demonstrate that pU(S)3 and MDV-specific phosphoprotein pp38 represent a kinase-substrate pair and that growth impairment in the absence of pU(S)3 is caused by the absence of kinase activity. The unaltered disruption of F-actin by the K220A pU(S)3 mutant suggests that F-actin disassembly is unrelated to MDV growth restrictions in the absence of the unique-short protein kinase.
Figures


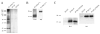
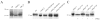

Similar articles
-
The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown.J Virol. 2005 Apr;79(7):3987-97. doi: 10.1128/JVI.79.7.3987-3997.2005. J Virol. 2005. PMID: 15767401 Free PMC article.
-
Role of Marek's Disease Virus (MDV)-Encoded US3 Serine/Threonine Protein Kinase in Regulating MDV Meq and Cellular CREB Phosphorylation.J Virol. 2020 Aug 17;94(17):e00892-20. doi: 10.1128/JVI.00892-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581093 Free PMC article.
-
Identification of a unique Marek's disease virus gene which encodes a 38-kilodalton phosphoprotein and is expressed in both lytically infected cells and latently infected lymphoblastoid tumor cells.J Virol. 1992 Jan;66(1):85-94. doi: 10.1128/JVI.66.1.85-94.1992. J Virol. 1992. PMID: 1309266 Free PMC article.
-
Latency and tumorigenesis in Marek's disease.Avian Dis. 2013 Jun;57(2 Suppl):360-5. doi: 10.1637/10470-121712-Reg.1. Avian Dis. 2013. PMID: 23901747 Review.
-
Viral serine/threonine protein kinases.J Virol. 2011 Feb;85(3):1158-73. doi: 10.1128/JVI.01369-10. Epub 2010 Nov 17. J Virol. 2011. PMID: 21084474 Free PMC article. Review.
Cited by
-
Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases.Curr Top Microbiol Immunol. 2010;342:79-98. doi: 10.1007/82_2009_7. Curr Top Microbiol Immunol. 2010. PMID: 20186610 Free PMC article. Review.
-
Marek's disease virus US3 protein kinase phosphorylates chicken HDAC 1 and 2 and regulates viral replication and pathogenesis.PLoS Pathog. 2021 Feb 17;17(2):e1009307. doi: 10.1371/journal.ppat.1009307. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33596269 Free PMC article.
-
Fully Attenuated meq and pp38 Double Gene Deletion Mutant Virus Confers Superior Immunological Protection against Highly Virulent Marek's Disease Virus Infection.Microbiol Spectr. 2022 Dec 21;10(6):e0287122. doi: 10.1128/spectrum.02871-22. Epub 2022 Nov 9. Microbiol Spectr. 2022. PMID: 36350141 Free PMC article.
-
Rho'ing in and out of cells: viral interactions with Rho GTPase signaling.Small GTPases. 2014;5:e28318. doi: 10.4161/sgtp.28318. Epub 2014 Mar 24. Small GTPases. 2014. PMID: 24691164 Free PMC article. Review.
-
Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3.Virology. 2010 Feb 5;397(1):23-33. doi: 10.1016/j.virol.2009.11.012. Epub 2009 Nov 28. Virology. 2010. PMID: 19945726 Free PMC article.
References
-
- Baigent SJ, Ross LJ, Davison TF. Differential susceptibility to Marek’s disease is associated with differences in number, but not phenotype or location, of pp38+ lymphocytes. J Gen Virol. 1998;79:2795–2802. - PubMed
-
- Calnek BW. Marek’s disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

