Protein phosphatase 2A is a negative regulator of transforming growth factor-beta1-induced TAK1 activation in mesangial cells
- PMID: 18299321
- PMCID: PMC2447645
- DOI: 10.1074/jbc.M801263200
Protein phosphatase 2A is a negative regulator of transforming growth factor-beta1-induced TAK1 activation in mesangial cells
Abstract
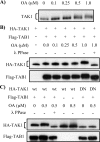
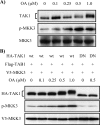
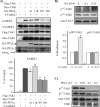
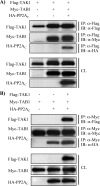
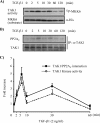
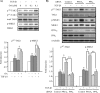
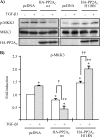
TAK1 (transforming growth factor (TGF)-beta-activated kinase 1) is a serine/threonine kinase that is rapidly activated by TGF-beta1 and plays a vital function in its signal transduction. Once TAK1 is activated, efficient down-regulation of TAK1 activity is important to prevent excessive TGF-beta1 responses. The regulatory mechanism of TAK1 inactivation following TGF-beta1 stimulation has not been elucidated. Here we demonstrate that protein phosphatase 2A (PP2A) plays a pivotal role as a negative regulator of TAK1 activation in response to TGF-beta1 in mesangial cells. Treatment with okadaic acid (OA) induces autophosphorylation of Thr-187 in the activation loop of TAK1. In vitro dephosphorylation assay suggests that Thr-187 in TAK1 is a major dephosphorylation target of PP2A. TGF-beta1 stimulation rapidly activates TAK1 in a biphasic manner, indicating that TGF-beta1-induced TAK1 activation is tightly regulated. The association of PP2A(C) with TAK1 is enhanced in response to TGF-beta1 stimulation and closely parallels TGF-beta1-induced TAK1 activity. Attenuation of PP2A activity by OA treatment or targeted knockdown of PP2A(C) with small interfering RNA enhances TGF-beta1-induced phosphorylation of TAK1 at Thr-187 and MKK3 (MAPK kinase 3). Endogenous TAK1 co-precipitates with PP2A(C) but not PP6(C), another OA-sensitive protein phosphatase, and knockdown of PP6(C) by small interfering RNA does not affect TGF-beta1-induced phosphorylation of TAK1 at Thr-187 and MKK3. Moreover, ectopic expression of phosphatase-deficient PP2A(C) enhances TAK1-mediated MKK3 phosphorylation by TGF-beta1 stimulation, whereas the expression of wild-type PP2A(C) suppresses the MKK3 phosphorylation. Taken together, our data indicate that PP2A functions as a negative regulator in TGF-beta1-induced TAK1 activation.
Figures

Similar articles
-
Transforming growth factor-beta (TGF-beta1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-beta receptor kinase activity in mesangial cells.J Biol Chem. 2009 Aug 14;284(33):22285-22296. doi: 10.1074/jbc.M109.007146. Epub 2009 Jun 25. J Biol Chem. 2009. PMID: 19556242 Free PMC article.
-
TGF-beta-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-beta1-induced MKK3-p38 MAPK activation and stimulation of type I collagen.Am J Physiol Renal Physiol. 2007 May;292(5):F1471-8. doi: 10.1152/ajprenal.00485.2006. Epub 2007 Feb 13. Am J Physiol Renal Physiol. 2007. PMID: 17299140
-
Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway.J Biol Chem. 2006 Dec 29;281(52):39891-6. doi: 10.1074/jbc.M608155200. Epub 2006 Nov 1. J Biol Chem. 2006. PMID: 17079228 Free PMC article.
-
[Transforming growth factor-β-activated kinase 1 and pathological myocardial hypertrophy].Sheng Li Xue Bao. 2020 Aug 25;72(4):499-505. Sheng Li Xue Bao. 2020. PMID: 32820312 Review. Chinese.
-
Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias.Cancer Metastasis Rev. 2008 Jun;27(2):159-68. doi: 10.1007/s10555-008-9119-x. Cancer Metastasis Rev. 2008. PMID: 18213449 Review.
Cited by
-
Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes.Nat Commun. 2019 Oct 4;10(1):4523. doi: 10.1038/s41467-019-12433-w. Nat Commun. 2019. PMID: 31586053 Free PMC article.
-
Interleukin-1 and TRAF6-dependent activation of TAK1 in the absence of TAB2 and TAB3.Biochem J. 2017 Jun 26;474(13):2235-2248. doi: 10.1042/BCJ20170288. Biochem J. 2017. PMID: 28507161 Free PMC article.
-
Transforming growth factor (TGF)-β-activated kinase 1 (TAK1) activation requires phosphorylation of serine 412 by protein kinase A catalytic subunit α (PKACα) and X-linked protein kinase (PRKX).J Biol Chem. 2014 Aug 29;289(35):24226-37. doi: 10.1074/jbc.M114.559963. Epub 2014 Jul 15. J Biol Chem. 2014. PMID: 25028512 Free PMC article.
-
TGF-β signaling via TAK1 pathway: role in kidney fibrosis.Semin Nephrol. 2012 May;32(3):244-52. doi: 10.1016/j.semnephrol.2012.04.003. Semin Nephrol. 2012. PMID: 22835455 Free PMC article. Review.
-
TIPE2 (Tumor Necrosis Factor α-induced Protein 8-like 2) Is a Novel Negative Regulator of TAK1 Signal.J Biol Chem. 2016 Oct 21;291(43):22650-22660. doi: 10.1074/jbc.M116.733451. Epub 2016 Sep 6. J Biol Chem. 2016. PMID: 27601471 Free PMC article.
References
-
- Heldin, C. H., Miyazono, K., and ten Dijke, P. (1997) Nature 390 465-471 - PubMed
-
- Massague, J. (1998) Annu. Rev. Biochem. 67 753-791 - PubMed
-
- Border, W. A., and Noble, N. A. (1994) N. Engl. J. Med. 331 1286-1292 - PubMed
-
- Cheng, J., and Grande, J. P. (2002) Exp. Biol. Med. 227 943-956 - PubMed
-
- Massague, J., Blain, S. W., and Lo, R. S. (2000) Cell 103 295-309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

