Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming
- PMID: 18295576
- PMCID: PMC4142810
- DOI: 10.1016/j.cell.2008.01.015
Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming
Abstract
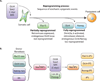
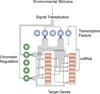
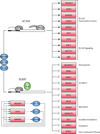
Reprogramming of somatic cells to a pluripotent embryonic stem cell-like state has been achieved by nuclear transplantation of a somatic nucleus into an enucleated egg and most recently by introducing defined transcription factors into somatic cells. Nuclear reprogramming is of great medical interest, as it has the potential to generate a source of patient-specific cells. Here, we review strategies to reprogram somatic cells to a pluripotent embryonic state and discuss our understanding of the molecular mechanisms of reprogramming based on recent insights into the regulatory circuitry of the pluripotent state.
Figures



Similar articles
-
Stem cells, pluripotency and nuclear reprogramming.J Thromb Haemost. 2009 Jul;7 Suppl 1:21-3. doi: 10.1111/j.1538-7836.2009.03418.x. J Thromb Haemost. 2009. PMID: 19630760 Review.
-
Reprogramming somatic cells towards pluripotency by cellular fusion.Curr Opin Genet Dev. 2012 Oct;22(5):459-65. doi: 10.1016/j.gde.2012.07.005. Epub 2012 Aug 3. Curr Opin Genet Dev. 2012. PMID: 22868176 Review.
-
Faithful reprogramming to pluripotency in mammals - what does nuclear transfer teach us?Int J Dev Biol. 2010;54(11-12):1609-21. doi: 10.1387/ijdb.103195jm. Int J Dev Biol. 2010. PMID: 21404182 Review.
-
Reprogramming of two somatic nuclei in the same ooplasm leads to pluripotent embryonic stem cells.Stem Cells. 2013 Nov;31(11):2343-53. doi: 10.1002/stem.1497. Stem Cells. 2013. PMID: 23922292
-
The role of the reprogramming method and pluripotency state in gamete differentiation from patient-specific human pluripotent stem cells.Mol Hum Reprod. 2018 Apr 1;24(4):173-184. doi: 10.1093/molehr/gay007. Mol Hum Reprod. 2018. PMID: 29471503
Cited by
-
OCT4/SOX2-independent Nanog autorepression modulates heterogeneous Nanog gene expression in mouse ES cells.EMBO J. 2012 Dec 12;31(24):4547-62. doi: 10.1038/emboj.2012.321. Epub 2012 Nov 23. EMBO J. 2012. PMID: 23178592 Free PMC article.
-
Simultaneous and quantitative monitoring transcription factors in human embryonic stem cell differentiation using mass spectrometry-based targeted proteomics.Anal Bioanal Chem. 2021 Mar;413(8):2081-2089. doi: 10.1007/s00216-021-03160-7. Epub 2021 Mar 2. Anal Bioanal Chem. 2021. PMID: 33655347
-
Ectopic expression of OCT4B1 Decreases Fertility Rate and Changes Sperm Parameters in Transgenic Mice.Iran J Biotechnol. 2022 Jul 1;20(3):e3019. doi: 10.30498/ijb.2022.278266.3019. eCollection 2022 Jul. Iran J Biotechnol. 2022. PMID: 36381279 Free PMC article.
-
SNF5 is an essential executor of epigenetic regulation during differentiation.PLoS Genet. 2013 Apr;9(4):e1003459. doi: 10.1371/journal.pgen.1003459. Epub 2013 Apr 25. PLoS Genet. 2013. PMID: 23637628 Free PMC article.
-
Induced neuronal cells: how to make and define a neuron.Cell Stem Cell. 2011 Dec 2;9(6):517-25. doi: 10.1016/j.stem.2011.11.015. Cell Stem Cell. 2011. PMID: 22136927 Free PMC article. Review.
References
-
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
-
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

