Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects
- PMID: 18294327
- PMCID: PMC2291369
- DOI: 10.1111/j.1365-2125.2007.03084.x
Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects
Abstract
What is already known about this subject: * Voriconazole, a broad-spectrum antifungal drug, is a substrate and inhibitor of CYP2C19 and CYP3A4 isozymes. * Ethinyl oestradiol and norethindrone, components of the combination oral contraceptive drug Ortho-Novum 1/35, also are substrates of cytochrome P450 CYP2C19 and CYP3A4 isozymes. * Because co-administration of voriconazole and Ortho-Novum 1/35 could potentially result in pharmacokinetic interactions that increase systemic exposure of one or both drugs to unsafe levels, clinical studies are needed to define better the two-way pharmacokinetic interaction between these drugs.
What this study adds: * Although co-administered voriconazole and oral contraceptive did result in increased systemic exposures of all three drugs relative to respective monotherapy, co-administered treatment was generally safe and well tolerated. * It is recommended, however, that patients receiving co-administered voriconazole and oral contraceptives be monitored for the development of adverse events commonly associated with these medications.
Aim: To assess the two-way pharmacokinetic interaction between voriconazole and Ortho-Novum 1/35, an oral contraceptive containing norethindrone 1 mg and ethinyl oestradiol 35 microg.
Methods: In this open-label, three-period, fixed-sequence study, 16 healthy females received voriconazole (400 mg q12 h, day 1; 200 mg q12 h, days 2-4) (period 1), oral contraceptive (q24 h, days 12-32) (period 2), and combination voriconazole (400 mg q12 h, day 57; 200 mg q12 h, days 58-60) and oral contraceptive (q24 h, days 40-60) (period 3).
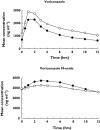
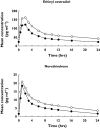
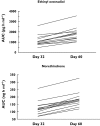
Results: Voriconazole geometric mean AUC(tau) and C(max) increased 46% (12 682-18 495 ng h ml(-1); 90% confidence interval [CI] 32, 61) and 14% (2485-2840 ng ml(-1); 90% CI 3, 27), respectively, when co-administered with oral contraceptive vs. voriconazole alone. Ethinyl oestradiol geometric mean AUC(tau) and C(max) increased 61% (1031-1657 ng h ml(-1); 90% CI 50, 72) and 36% (119-161 ng ml(-1); 90% CI 28, 45), respectively, and norethindrone geometric mean AUC(tau) and C(max) increased 53% (116-177 ng h ml(-1); 90% CI 44, 64) and 15% (18-20 ng ml(-1); 90% CI 3, 28), respectively, during voriconazole co-administration vs. oral contraceptive alone. Neither ethinyl oestradiol nor norethindrone levels were reduced in subjects following voriconazole co-administration. Adverse events (AEs) were generally mild, occurring less in subjects receiving voriconazole alone (36 events) vs. oral contraceptive alone (88 events) or combination treatment (68 events); four subjects experienced a severe AE.
Conclusions: Co-administration of voriconazole and oral contraceptive increased systemic exposures of all analytes relative to respective monotherapy. Although generally safe and well tolerated, it is recommended that patients receiving co-administered voriconazole and oral contraceptive be monitored for development of AEs commonly associated with these medications.
Figures

Similar articles
-
Pharmacokinetic interactions of efavirenz and voriconazole in healthy volunteers.Br J Clin Pharmacol. 2008 Apr;65(4):523-30. doi: 10.1111/j.1365-2125.2007.03085.x. Epub 2008 Feb 20. Br J Clin Pharmacol. 2008. PMID: 18294336 Free PMC article.
-
Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3A4 inhibitor ritonavir.Clin Pharmacol Ther. 2006 Aug;80(2):126-35. doi: 10.1016/j.clpt.2006.04.004. Epub 2006 Jul 3. Clin Pharmacol Ther. 2006. PMID: 16890574 Clinical Trial.
-
Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens.Antimicrob Agents Chemother. 2002 Aug;46(8):2546-53. doi: 10.1128/AAC.46.8.2546-2553.2002. Antimicrob Agents Chemother. 2002. PMID: 12121931 Free PMC article. Clinical Trial.
-
Pharmacogenomics of the triazole antifungal agent voriconazole.Pharmacogenomics. 2011 Jun;12(6):861-72. doi: 10.2217/pgs.11.18. Pharmacogenomics. 2011. PMID: 21692616 Review.
-
Pharmacokinetic/pharmacodynamic profile of voriconazole.Clin Pharmacokinet. 2006;45(7):649-63. doi: 10.2165/00003088-200645070-00002. Clin Pharmacokinet. 2006. PMID: 16802848 Review.
Cited by
-
Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults.Antimicrob Agents Chemother. 2011 Dec;55(12):5770-9. doi: 10.1128/AAC.00531-11. Epub 2011 Oct 3. Antimicrob Agents Chemother. 2011. PMID: 21968355 Free PMC article. Clinical Trial.
-
Bronchopulmonary disposition of intravenous voriconazole and anidulafungin given in combination to healthy adults.Antimicrob Agents Chemother. 2009 Dec;53(12):5102-7. doi: 10.1128/AAC.01042-09. Epub 2009 Sep 21. Antimicrob Agents Chemother. 2009. PMID: 19770284 Free PMC article. Clinical Trial.
-
Role of CYP3A in Oral Contraceptives Clearance.Clin Transl Sci. 2018 May;11(3):251-260. doi: 10.1111/cts.12499. Epub 2017 Oct 6. Clin Transl Sci. 2018. PMID: 28986954 Free PMC article. Review. No abstract available.
-
Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised adolescents and healthy adults.Antimicrob Agents Chemother. 2011 Dec;55(12):5780-9. doi: 10.1128/AAC.05010-11. Epub 2011 Sep 12. Antimicrob Agents Chemother. 2011. PMID: 21911570 Free PMC article. Clinical Trial.
-
Pharmacokinetic interaction of voriconazole and clarithromycin in Pakistani healthy male volunteers: a single dose, randomized, crossover, open-label study.Front Pharmacol. 2023 Jun 9;14:1134803. doi: 10.3389/fphar.2023.1134803. eCollection 2023. Front Pharmacol. 2023. PMID: 37361220 Free PMC article.
References
-
- Pfizer Inc. Vfend®. US Prescribing Information. New York, NY: Pfizer Inc.; 2006.
-
- Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. - PubMed
-
- Ortho-McNeil Pharmaceutical, Inc. Ortho-Novum® 1/35. US Prescribing Information. Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc.; 2006.
-
- Goldzieher JW. Pharmacokinetics and metabolism of ethinyl estrogens. In: Goldzieher JW, Fotherby K, editors. Pharmacology of the Contraceptive Steroids. New York: Raven Press; 1994. pp. 127–51.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

