Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development
- PMID: 18287232
- PMCID: PMC2293049
- DOI: 10.1128/JVI.02541-07
Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development
Abstract
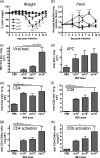
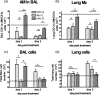
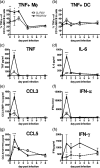
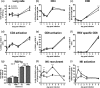
Macrophages are abundant in the lower respiratory tract. They play a central role in the innate response to infection but may also modulate excessive inflammation. Both macrophages and ciliated epithelial cells respond to infection by releasing soluble mediators, leading to the recruitment of innate and adaptive effector cells. To study the role of lung macrophages in acute respiratory viral infection, we depleted them by the inhalation of clodronate liposomes in an established mouse model of respiratory syncytial virus (RSV) disease. Infection caused an immediate local release of inflammatory cytokines and chemokines, peaking on day 1, which was virtually abolished by clodronate liposome treatment. Macrophage depletion inhibited the activation (days 1 to 2) and recruitment (day 4) of natural killer (NK) cells and enhanced peak viral load in the lung (day 4). However, macrophage depletion did not affect the recruitment of activated CD4 or CD8 T cells, weight loss, or virus-induced changes in lung function. Therefore, lung macrophages play a central role in the early responses to viral infection but have remarkably little effect on the adaptive response occurring at the time of peak disease severity.
Figures
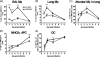
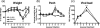
Similar articles
-
Cellular Immune Correlates Preventing Disease Against Respiratory Syncytial Virus by Vaccination with Virus-Like Nanoparticles Carrying Fusion Proteins.J Biomed Nanotechnol. 2017 Jan;13(1):84-98. doi: 10.1166/jbn.2017.2341. J Biomed Nanotechnol. 2017. PMID: 29302248 Free PMC article.
-
Engystol reduces onset of experimental respiratory syncytial virus-induced respiratory inflammation in mice by modulating macrophage phagocytic capacity.PLoS One. 2018 Apr 19;13(4):e0195822. doi: 10.1371/journal.pone.0195822. eCollection 2018. PLoS One. 2018. PMID: 29672626 Free PMC article.
-
Innate and adaptive cellular phenotypes contributing to pulmonary disease in mice after respiratory syncytial virus immunization and infection.Virology. 2015 Nov;485:36-46. doi: 10.1016/j.virol.2015.07.001. Epub 2015 Jul 18. Virology. 2015. PMID: 26196232 Free PMC article.
-
Human Respiratory Syncytial Virus: Role of Innate Immunity in Clearance and Disease Progression.Viral Immunol. 2016 Jan-Feb;29(1):11-26. doi: 10.1089/vim.2015.0098. Epub 2015 Dec 17. Viral Immunol. 2016. PMID: 26679242 Review.
-
Chemokine regulation of inflammation during respiratory syncytial virus infection.F1000Res. 2019 Oct 31;8:F1000 Faculty Rev-1837. doi: 10.12688/f1000research.20061.1. eCollection 2019. F1000Res. 2019. PMID: 31723414 Free PMC article. Review.
Cited by
-
Investigation of immune cells on elimination of pulmonary-Infected COVID-19 and important role of innate immunity, phagocytes.Rev Med Virol. 2021 Mar;31(2):e2158. doi: 10.1002/rmv.2158. Epub 2020 Sep 18. Rev Med Virol. 2021. PMID: 32946131 Free PMC article. Review.
-
Partial Attenuation of Respiratory Syncytial Virus with a Deletion of a Small Hydrophobic Gene Is Associated with Elevated Interleukin-1β Responses.J Virol. 2015 Sep;89(17):8974-81. doi: 10.1128/JVI.01070-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085154 Free PMC article.
-
Respiratory epithelial cells as master communicators during viral infections.Curr Clin Microbiol Rep. 2019 Mar;6(1):10-17. doi: 10.1007/s40588-019-0111-8. Epub 2019 Feb 13. Curr Clin Microbiol Rep. 2019. PMID: 31592409 Free PMC article.
-
Immunity Cell Responses to RSV and the Role of Antiviral Inhibitors: A Systematic Review.Infect Drug Resist. 2022 Dec 14;15:7413-7430. doi: 10.2147/IDR.S387479. eCollection 2022. Infect Drug Resist. 2022. PMID: 36540102 Free PMC article. Review.
-
Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation.Cells. 2021 Oct 12;10(10):2720. doi: 10.3390/cells10102720. Cells. 2021. PMID: 34685700 Free PMC article. Review.
References
-
- Becker, S., J. Quay, and J. Soukup. 1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1474307-4312. - PubMed
-
- Durbin, J. E., T. R. Johnson, R. K. Durbin, S. E. Mertz, R. A. Morotti, R. S. Peebles, and B. S. Graham. 2002. The role of IFN in respiratory syncytial virus pathogenesis. J. Immunol. 1682944-2952. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

