NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-kappaB activation
- PMID: 18287044
- PMCID: PMC2268578
- DOI: 10.1073/pnas.0712313105
NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-kappaB activation
Abstract
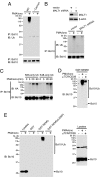
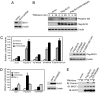
The mechanism by which the Carma1-Bcl10-MALT1 (CBM) complex couples T cell antigen receptor (TCR) signaling to IkappaB kinase (IKK) and NF-kappaB activation is not known. Here, we show that Bcl10 undergoes K63-linked polyubiquitination in response to T cell activation and subsequently binds NEMO, the regulatory subunit of IKK. This interaction requires the ubiquitin-binding activity of NEMO. The sites of Bcl10 ubiquitination were mapped to K31 and K63. Mutation of these residues did not affect TCR signaling-induced CBM complex assembly but prevented Bcl10 ubiquitination, NEMO binding, and NF-kappaB activation. Therefore, the regulated ubiquitination of Bcl10 and its recognition by NEMO are a critical link between the CBM complex, IKK recruitment, and NF-kappaB activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



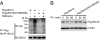
Similar articles
-
Molecular Determinants of Scaffold-induced Linear Ubiquitinylation of B Cell Lymphoma/Leukemia 10 (Bcl10) during T Cell Receptor and Oncogenic Caspase Recruitment Domain-containing Protein 11 (CARD11) Signaling.J Biol Chem. 2016 Dec 9;291(50):25921-25936. doi: 10.1074/jbc.M116.754028. Epub 2016 Oct 24. J Biol Chem. 2016. PMID: 27777308 Free PMC article.
-
Malt1 ubiquitination triggers NF-kappaB signaling upon T-cell activation.EMBO J. 2007 Nov 14;26(22):4634-45. doi: 10.1038/sj.emboj.7601897. Epub 2007 Oct 18. EMBO J. 2007. PMID: 17948050 Free PMC article.
-
T cell receptor signals to NF-κB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome.Sci Signal. 2014 May 13;7(325):ra45. doi: 10.1126/scisignal.2004882. Sci Signal. 2014. PMID: 24825920 Free PMC article.
-
Critical protein-protein interactions within the CARMA1-BCL10-MALT1 complex: Take-home points for the cell biologist.Cell Immunol. 2020 Sep;355:104158. doi: 10.1016/j.cellimm.2020.104158. Epub 2020 Jul 7. Cell Immunol. 2020. PMID: 32721634 Review.
-
Ubiquitination in signaling to and activation of IKK.Immunol Rev. 2012 Mar;246(1):95-106. doi: 10.1111/j.1600-065X.2012.01108.x. Immunol Rev. 2012. PMID: 22435549 Free PMC article. Review.
Cited by
-
Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation.Mol Cell Biol. 2008 May;28(10):3538-47. doi: 10.1128/MCB.02098-07. Epub 2008 Mar 17. Mol Cell Biol. 2008. PMID: 18347055 Free PMC article.
-
Identification of Transcripts with Shared Roles in the Pathogenesis of Postmenopausal Osteoporosis and Cardiovascular Disease.Int J Mol Sci. 2024 May 20;25(10):5554. doi: 10.3390/ijms25105554. Int J Mol Sci. 2024. PMID: 38791593 Free PMC article.
-
NKT sublineage specification and survival requires the ubiquitin-modifying enzyme TNFAIP3/A20.J Exp Med. 2016 Sep 19;213(10):1973-81. doi: 10.1084/jem.20151065. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551157 Free PMC article.
-
A new look at T cell receptor signaling to nuclear factor-κB.Trends Immunol. 2013 Jun;34(6):269-81. doi: 10.1016/j.it.2013.02.002. Epub 2013 Mar 7. Trends Immunol. 2013. PMID: 23474202 Free PMC article. Review.
-
Lipopolysaccharide-induced activation of NF-κB non-canonical pathway requires BCL10 serine 138 and NIK phosphorylations.Exp Cell Res. 2010 Nov 15;316(19):3317-27. doi: 10.1016/j.yexcr.2010.05.004. Epub 2010 May 11. Exp Cell Res. 2010. PMID: 20466000 Free PMC article.
References
-
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. - PubMed
-
- Clements JL, Boerth NJ, Lee JR, Koretzky GA. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu Rev Immunol. 1999;17:89–108. - PubMed
-
- Weil R, Israel A. Deciphering the pathway from the TCR to NF-κB. Cell Death Differ. 2006;13:826–833. - PubMed
-
- Thome M, Tschopp J. TCR-induced NF-κB activation: A crucial role for Carma1, Bcl10, and MALT1. Trends Immunol. 2003;24:419–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

