Genetic analysis of the role of proteolysis in the activation of latent myostatin
- PMID: 18286185
- PMCID: PMC2237902
- DOI: 10.1371/journal.pone.0001628
Genetic analysis of the role of proteolysis in the activation of latent myostatin
Abstract
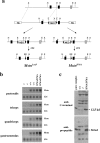
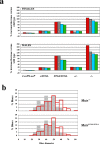
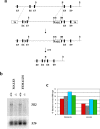
Myostatin is a secreted protein that normally acts to limit skeletal muscle growth. As a result, there is considerable interest in developing agents capable of blocking myostatin activity, as such agents could have widespread applications for the treatment of muscle degenerative and wasting conditions. Myostatin normally exists in an inactive state in which the mature C-terminal portion of the molecule is bound non-covalently to its N-terminal propeptide. We previously showed that this latent complex can be activated in vitro by cleavage of the propeptide with members of the bone morphogenetic protein-1/tolloid (BMP-1/TLD) family of metalloproteases. Here, I show that mice engineered to carry a germline point mutation rendering the propeptide protease-resistant exhibit increases in muscle mass approaching those seen in mice completely lacking myostatin. Mice homozygous for the point mutation have increased muscling even though their circulating levels of myostatin protein are dramatically increased, consistent with an inability of myostatin to be activated from its latent state. Furthermore, I show that a loss-of-function mutation in Tll2, which encodes one member of this protease family, has a small, but significant, effect on muscle mass, implying that its function is likely redundant with those of other family members. These findings provide genetic support for the hypothesis that proteolytic cleavage of the propeptide by BMP-1/TLD proteases plays a critical role in the activation of latent myostatin in vivo and suggest that targeting the activities of these proteases may be an effective therapeutic strategy for enhancing muscle growth in clinical settings of muscle loss and degeneration.
Conflict of interest statement
Figures
Similar articles
-
Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15842-6. doi: 10.1073/pnas.2534946100. Epub 2003 Dec 11. Proc Natl Acad Sci U S A. 2003. PMID: 14671324 Free PMC article.
-
Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis.Dev Biol. 1999 Sep 15;213(2):283-300. doi: 10.1006/dbio.1999.9383. Dev Biol. 1999. PMID: 10479448
-
Developmental roles of the BMP1/TLD metalloproteinases.Birth Defects Res C Embryo Today. 2006 Mar;78(1):47-68. doi: 10.1002/bdrc.20060. Birth Defects Res C Embryo Today. 2006. PMID: 16622848 Review.
-
Bone morphogenetic protein-1 processes probiglycan.J Biol Chem. 2000 Sep 29;275(39):30504-11. doi: 10.1074/jbc.M004846200. J Biol Chem. 2000. PMID: 10896944
-
The role of myostatin and bone morphogenetic proteins in muscular disorders.Expert Opin Biol Ther. 2006 Feb;6(2):147-54. doi: 10.1517/14712598.6.2.147. Expert Opin Biol Ther. 2006. PMID: 16436040 Review.
Cited by
-
Skeletal muscle expression of bone morphogenetic protein-1 and tolloid-like-1 extracellular proteases in different fiber types and in response to unloading, food deprivation and differentiation.J Physiol Sci. 2010 Sep;60(5):343-52. doi: 10.1007/s12576-010-0104-0. Epub 2010 Jul 24. J Physiol Sci. 2010. PMID: 20658214 Free PMC article.
-
Extracellular Regulation of Myostatin: A Molecular Rheostat for Muscle Mass.Immunol Endocr Metab Agents Med Chem. 2010;10:183-194. doi: 10.2174/187152210793663748. Immunol Endocr Metab Agents Med Chem. 2010. PMID: 21423813 Free PMC article.
-
Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2.FEBS J. 2013 Aug;280(16):3822-39. doi: 10.1111/febs.12377. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23829672 Free PMC article.
-
Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep.PLoS One. 2013;8(3):e58521. doi: 10.1371/journal.pone.0058521. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23526994 Free PMC article.
-
Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice.Hum Mol Genet. 2014 Jun 15;23(12):3085-101. doi: 10.1093/hmg/ddu013. Epub 2014 Jan 12. Hum Mol Genet. 2014. PMID: 24419319 Free PMC article.
References
-
- Lee S-J. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. - PubMed
-
- McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-ß superfamily member. Nature. 1997;387:83–90. - PubMed
-
- Grobet L, Martin LJR, Poncelet D, Pirottin D, Brouwers B, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nature Genet. 1997;17:71–74. - PubMed
-
- Kambadur R, Sharma M, Smith TPL, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

