BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties
- PMID: 18281466
- PMCID: PMC2238674
- DOI: 10.1101/gad.1614408
BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties
Abstract
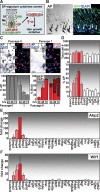
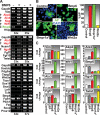
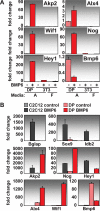
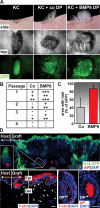
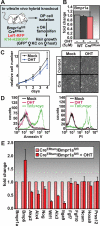
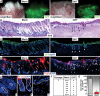
Hair follicle (HF) formation is initiated when epithelial stem cells receive cues from specialized mesenchymal dermal papilla (DP) cells. In culture, DP cells lose their HF-inducing properties, but during hair growth in vivo, they reside within the HF bulb and instruct surrounding epithelial progenitors to orchestrate the complex hair differentiation program. To gain insights into the molecular program that maintains DP cell fate, we previously purified DP cells and four neighboring populations and defined their cell-type-specific molecular signatures. Here, we exploit this information to show that the bulb microenvironment is rich in bone morphogenetic proteins (BMPs) that act on DP cells to maintain key signature features in vitro and hair-inducing activity in vivo. By employing a novel in vitro/in vivo hybrid knockout assay, we ablate BMP receptor 1a in purified DP cells. When DPs cannot receive BMP signals, they lose signature characteristics in vitro and fail to generate HFs when engrafted with epithelial stem cells in vivo. These results reveal that BMP signaling, in addition to its key role in epithelial stem cell maintenance and progenitor cell differentiation, is essential for DP cell function, and suggest that it is a critical feature of the complex epithelial-mesenchymal cross-talk necessary to make hair.
Figures


Similar articles
-
Molecular dissection of mesenchymal-epithelial interactions in the hair follicle.PLoS Biol. 2005 Nov;3(11):e331. doi: 10.1371/journal.pbio.0030331. Epub 2005 Sep 20. PLoS Biol. 2005. PMID: 16162033 Free PMC article.
-
Hair Follicle Terminal Differentiation Is Orchestrated by Distinct Early and Late Matrix Progenitors.Cell Rep. 2017 Apr 25;19(4):809-821. doi: 10.1016/j.celrep.2017.03.077. Cell Rep. 2017. PMID: 28445731 Free PMC article.
-
Assembling Composite Dermal Papilla Spheres with Adipose-derived Stem Cells to Enhance Hair Follicle Induction.Sci Rep. 2016 May 23;6:26436. doi: 10.1038/srep26436. Sci Rep. 2016. PMID: 27210831 Free PMC article.
-
The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle.Cold Spring Harb Perspect Med. 2014 Jul 1;4(7):a015180. doi: 10.1101/cshperspect.a015180. Cold Spring Harb Perspect Med. 2014. PMID: 24985131 Free PMC article. Review.
-
Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods.Skin Pharmacol Physiol. 2020;33(5):280-292. doi: 10.1159/000510152. Epub 2020 Oct 14. Skin Pharmacol Physiol. 2020. PMID: 33053562 Review.
Cited by
-
Wound healing and skin regeneration.Cold Spring Harb Perspect Med. 2015 Jan 5;5(1):a023267. doi: 10.1101/cshperspect.a023267. Cold Spring Harb Perspect Med. 2015. PMID: 25561722 Free PMC article. Review.
-
Frizzled6 deficiency disrupts the differentiation process of nail development.J Invest Dermatol. 2013 Aug;133(8):1990-7. doi: 10.1038/jid.2013.84. Epub 2013 Feb 25. J Invest Dermatol. 2013. PMID: 23439395 Free PMC article.
-
Recreation of a hair follicle regenerative microenvironment: Successes and pitfalls.Bioeng Transl Med. 2021 Jun 23;7(1):e10235. doi: 10.1002/btm2.10235. eCollection 2022 Jan. Bioeng Transl Med. 2021. PMID: 35079623 Free PMC article. Review.
-
miR-195-5p Regulates Hair Follicle Inductivity of Dermal Papilla Cells by Suppressing Wnt/β-Catenin Activation.Biomed Res Int. 2018 Apr 22;2018:4924356. doi: 10.1155/2018/4924356. eCollection 2018. Biomed Res Int. 2018. PMID: 29850524 Free PMC article.
-
Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration.Cell. 2011 Jun 10;145(6):941-955. doi: 10.1016/j.cell.2011.05.004. Cell. 2011. PMID: 21663796 Free PMC article.
References
-
- Akiyama S., Katagiri T., Namiki M., Yamaji N., Yamamoto N., Miyama K., Shibuya H., Ueno N., Wozney J.M., Suda T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp. Cell Res. 1997;235:362–369. - PubMed
-
- Alonso L., Fuchs E. The hair cycle. J. Cell Sci. 2006;119:391–393. - PubMed
-
- Andl T., Ahn K., Kairo A., Chu E.Y., Wine-Lee L., Reddy S.T., Croft N.J., Cebra-Thomas J.A., Metzger D., Chambon P., et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Blanpain C., Lowry W.E., Geoghegan A., Polak L., Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
