The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice
- PMID: 18272579
- PMCID: PMC2293007
- DOI: 10.1128/JVI.02313-07
The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice
Abstract
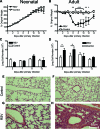
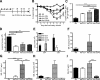
Respiratory syncytial virus (RSV) is the major cause of infantile bronchiolitis and hospitalization. Severe RSV disease is associated with the development of wheezing in later life. In a mouse model of the delayed effects of RSV, the age at primary infection determines responses to reinfection in adulthood. During primary RSV infection, neonatal BALB/c mice developed only mild disease and recruited CD8 cells that were defective in gamma interferon production. Secondary reinfection of neonatally primed mice caused enhanced inflammation and profuse lung T-cell recruitment. CD4 cell depletion during secondary RSV challenge attenuated disease (measured by weight loss); depletion of CD8 cells also markedly attenuated disease severity but enhanced lung eosinophilia, and depletion of both CD4 and CD8 cells together completely abrogated weight loss. Depletion of CD8 (but not CD4) cells during primary neonatal infection was protective against weight loss during adult challenge. Therefore, T cells, in particular CD8 T cells, play a central role in the outcome of neonatal infection by enhancing disease during secondary challenge. These findings demonstrate a crucial role for T cells in the regulation of immune responses after neonatal infection.
Figures

Similar articles
-
Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge.Clin Exp Immunol. 2008 Aug;153(2):277-88. doi: 10.1111/j.1365-2249.2008.03591.x. Epub 2008 Jun 28. Clin Exp Immunol. 2008. PMID: 18549446 Free PMC article.
-
STAT4 deficiency fails to induce lung Th2 or Th17 immunity following primary or secondary respiratory syncytial virus (RSV) challenge but enhances the lung RSV-specific CD8+ T cell immune response to secondary challenge.J Virol. 2014 Sep 1;88(17):9655-72. doi: 10.1128/JVI.03299-13. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920804 Free PMC article.
-
Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection.J Exp Med. 1997 Aug 4;186(3):421-32. doi: 10.1084/jem.186.3.421. J Exp Med. 1997. PMID: 9236194 Free PMC article.
-
RSV-induced immunopathology: dynamic interplay between the virus and host immune response.Virology. 2002 Apr 10;295(2):203-7. doi: 10.1006/viro.2002.1382. Virology. 2002. PMID: 12033778 Review. No abstract available.
-
Respiratory syncytial virus and T cells: interplay between the virus and the host adaptive immune system.Proc Am Thorac Soc. 2005;2(2):141-6. doi: 10.1513/pats.200503-022AW. Proc Am Thorac Soc. 2005. PMID: 16113482 Review.
Cited by
-
Pre-existing virus-specific CD8(+) T-cells provide protection against pneumovirus-induced disease in mice.Vaccine. 2012 Oct 5;30(45):6382-8. doi: 10.1016/j.vaccine.2012.08.027. Epub 2012 Aug 29. Vaccine. 2012. PMID: 22940382 Free PMC article.
-
Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology.Clin Microbiol Rev. 2010 Jan;23(1):74-98. doi: 10.1128/CMR.00032-09. Clin Microbiol Rev. 2010. PMID: 20065326 Free PMC article. Review.
-
The use of a neonatal mouse model to study respiratory syncytial virus infections.Expert Rev Anti Infect Ther. 2010 Dec;8(12):1371-80. doi: 10.1586/eri.10.125. Expert Rev Anti Infect Ther. 2010. PMID: 21133663 Free PMC article. Review.
-
Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence.mBio. 2020 Feb 18;11(1):e03236-19. doi: 10.1128/mBio.03236-19. mBio. 2020. PMID: 32071269 Free PMC article.
-
Respiratory Syncytial Virus Infection Upregulates NLRC5 and Major Histocompatibility Complex Class I Expression through RIG-I Induction in Airway Epithelial Cells.J Virol. 2015 Aug;89(15):7636-45. doi: 10.1128/JVI.00349-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972545 Free PMC article.
References
-
- Adkins, B., C. LeClerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4553-564. - PubMed
-
- Bisgaard, H., M. N. Hermansen, F. Buchvald, L. Loland, L. B. Halkjaer, K. Bonnelykke, M. Brasholt, A. Heltberg, N. H. Vissing, S. V. Thorsen, M. Stage, and C. B. Pipper. 2007. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 3571487-1495. - PubMed
-
- Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 854-60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

