Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn
- PMID: 18272495
- PMCID: PMC2268137
- DOI: 10.1073/pnas.0708960105
Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn
Abstract
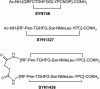
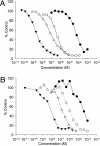
The neonatal Fc receptor FcRn provides IgG molecules with their characteristically long half-lives in vivo by protecting them from intracellular catabolism and then returning them to the extracellular space. Other investigators have demonstrated that mice lacking FcRn are protected from induction of various autoimmune diseases, presumably because of the accelerated catabolism of pathogenic IgGs in the animals. Therefore, targeting FcRn with a specific inhibitor may represent a unique approach for the treatment of autoimmune disease or other diseases where the reduction of pathogenic IgG will have a therapeutic benefit. Using phage display peptide libraries, we screened for ligands that bound to human FcRn (hFcRn) and discovered a consensus peptide sequence that binds to hFcRn and inhibits the binding of human IgG (hIgG) in vitro. Chemical optimization of the phage-identified sequences yielded the 26-amino acid peptide dimer SYN1436, which is capable of potent in vitro inhibition of the hIgG-hFcRn interaction. Administration of SYN1436 to mice transgenic for hFcRn induced an increase in the rate of catabolism of hIgG in a dose-dependent manner. Treatment of cynomolgus monkeys with SYN1436 led to a reduction of IgG by up to 80% without reducing serum albumin levels that also binds to FcRn. SYN1436 and related peptides thus represent a previously uncharacterized family of potential therapeutic agents for the treatment of humorally mediated autoimmune and other diseases.
Conflict of interest statement
Conflict of interest statement: Syntonix has filed a patent application covering the peptides described herein.
Figures
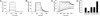

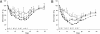
Similar articles
-
The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity.MAbs. 2015;7(2):331-43. doi: 10.1080/19420862.2015.1008353. MAbs. 2015. PMID: 25658443 Free PMC article.
-
Discovery and structure-activity relationships of small molecules that block the human immunoglobulin G-human neonatal Fc receptor (hIgG-hFcRn) protein-protein interaction.Bioorg Med Chem Lett. 2013 Mar 1;23(5):1253-6. doi: 10.1016/j.bmcl.2013.01.014. Epub 2013 Jan 11. Bioorg Med Chem Lett. 2013. PMID: 23375228
-
Qualification of a homogeneous cell-based neonatal Fc receptor (FcRn) binding assay and its application to studies on Fc functionality of IgG-based therapeutics.J Immunol Methods. 2013 Apr 30;390(1-2):81-91. doi: 10.1016/j.jim.2013.01.011. Epub 2013 Feb 4. J Immunol Methods. 2013. PMID: 23384837
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention.J Allergy Clin Immunol. 2020 Sep;146(3):467-478. doi: 10.1016/j.jaci.2020.07.015. J Allergy Clin Immunol. 2020. PMID: 32896307 Review.
Cited by
-
Targeting FcRn for the modulation of antibody dynamics.Mol Immunol. 2015 Oct;67(2 Pt A):131-41. doi: 10.1016/j.molimm.2015.02.007. Epub 2015 Mar 9. Mol Immunol. 2015. PMID: 25766596 Free PMC article. Review.
-
Regulation of immune responses by the neonatal fc receptor and its therapeutic implications.Front Immunol. 2015 Jan 5;5:664. doi: 10.3389/fimmu.2014.00664. eCollection 2014. Front Immunol. 2015. PMID: 25601863 Free PMC article. Review.
-
Neonatal Fc receptor and IgG-based therapeutics.MAbs. 2011 Sep-Oct;3(5):422-30. doi: 10.4161/mabs.3.5.16983. Epub 2011 Sep 1. MAbs. 2011. PMID: 22048693 Free PMC article. Review.
-
Role of Fc Receptors as a therapeutic target.Inflamm Allergy Drug Targets. 2009 Mar;8(1):80-6. doi: 10.2174/187152809787582525. Inflamm Allergy Drug Targets. 2009. PMID: 19275696 Free PMC article. Review.
-
Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice.PLoS One. 2014 Jul 24;9(7):e102566. doi: 10.1371/journal.pone.0102566. eCollection 2014. PLoS One. 2014. PMID: 25057984 Free PMC article.
References
-
- Sell S, Fahey JL. J Immunol. 1964;93:81–87. - PubMed
-
- Brambell FWR, Hemmings WA, Morris IG. Nature. 1964;203:1352. - PubMed
-
- Ghetie V, Ward ES. Annu Rev Immunol. 2000;18:739–766. - PubMed
-
- Roopenian DC, Akilesh S. Nat Rev Immunol. 2007;7:715–725. - PubMed
-
- Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Int Immunol. 1998;10:1289–1298. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

