Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset
- PMID: 18272486
- PMCID: PMC2268579
- DOI: 10.1073/pnas.0712394105
Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset
Abstract
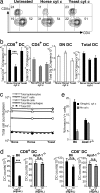
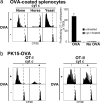
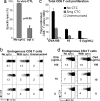
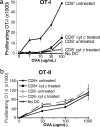
Cross-presentation as a fundamental pathway of activating CD8(+) T cells has been well established. So far the application of this concept in vivo is limited, and the mechanisms that specialize CD8(+) dendritic cells (DCs) for this task are not fully understood. Here we take advantage of the specific cytosolic export feature of cross-presenting DCs together with the property of cytosolic cytochrome c (cyt c) in initiating Apaf-1-dependent apoptosis selectively in cross-presenting DCs. A single i.v. injection of cyt c in B6 mice produced a 2- to 3-fold reduction in splenic CD8(+) DCs but not in Apaf-1-deficient mice. Functional studies both in vivo and in vitro showed that cyt c profoundly abrogated OVA-specific CD8(+) T cell proliferation through its apoptosis-inducing effect on cross-presenting DCs. More importantly, in vivo injection of cyt c abolished the induction of cytotoxic T lymphocytes to exogenous antigen and reduced subsequent immunity to tumor challenge. In addition, only a proportion of CD8(+) DCs that express abundant IL-12 and Toll-like receptor 3 were efficient cross-presenters. Our data support the hypothesis that cross-presentation in vivo requires cytosolic diversion of endocytosed proteins, conferring cross-presentation specialization to a proportion of CD8(+) DCs. We propose that DCs incapable of such transfer, even within the CD8(+) DC subset, are unable to cross-present. Our model opens an avenue to specifically target cross-presenting DCs in vivo for manipulating cytotoxic T lymphocyte responses toward infections, tumors, and transplants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
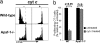
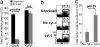
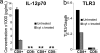
Similar articles
-
Administration of alpha-galactosylceramide impairs the survival of dendritic cell subpopulations in vivo.J Leukoc Biol. 2011 May;89(5):753-62. doi: 10.1189/jlb.0910480. Epub 2011 Feb 4. J Leukoc Biol. 2011. PMID: 21297009
-
CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo.J Exp Med. 2000 Dec 18;192(12):1685-96. doi: 10.1084/jem.192.12.1685. J Exp Med. 2000. PMID: 11120766 Free PMC article.
-
A Highly Active Form of XCL1/Lymphotactin Functions as an Effective Adjuvant to Recruit Cross-Presenting Dendritic Cells for Induction of Effector and Memory CD8+ T Cells.Front Immunol. 2018 Nov 27;9:2775. doi: 10.3389/fimmu.2018.02775. eCollection 2018. Front Immunol. 2018. PMID: 30542351 Free PMC article.
-
Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment.Front Immunol. 2018 Dec 20;9:3059. doi: 10.3389/fimmu.2018.03059. eCollection 2018. Front Immunol. 2018. PMID: 30619378 Free PMC article. Review.
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
Cited by
-
Experimental models to investigate the function of dendritic cell subsets: challenges and implications.Clin Exp Immunol. 2013 Feb;171(2):147-54. doi: 10.1111/cei.12027. Clin Exp Immunol. 2013. PMID: 23286941 Free PMC article. Review.
-
The best smellers make the best choosers: mate choice is affected by female chemosensory receptor gene diversity in a mammal.Proc Biol Sci. 2018 Dec 19;285(1893):20182426. doi: 10.1098/rspb.2018.2426. Proc Biol Sci. 2018. PMID: 30963892 Free PMC article.
-
Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma.Oncotarget. 2018 Apr 17;9(29):20681-20697. doi: 10.18632/oncotarget.25061. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755681 Free PMC article.
-
Sustained cross-presentation capacity of murine splenic dendritic cell subsets in vivo.Eur J Immunol. 2018 Jul;48(7):1164-1173. doi: 10.1002/eji.201747372. Epub 2018 May 17. Eur J Immunol. 2018. PMID: 29676785 Free PMC article.
-
Lipid peroxidation causes endosomal antigen release for cross-presentation.Sci Rep. 2016 Feb 24;6:22064. doi: 10.1038/srep22064. Sci Rep. 2016. PMID: 26907999 Free PMC article.
References
-
- Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. - PubMed
-
- Rodriguez A, et al. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. - PubMed
-
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. - PubMed
-
- Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. - PubMed
-
- Palmowski MJ, et al. Role of immunoproteasomes in cross-presentation. J Immunol. 2006;177:983–990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

