Hepatocyte growth factor regulates cyclooxygenase-2 expression via beta-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells
- PMID: 18245266
- PMCID: PMC2427436
- DOI: 10.1152/ajplung.00410.2007
Hepatocyte growth factor regulates cyclooxygenase-2 expression via beta-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells
Abstract
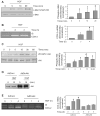
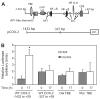
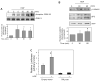
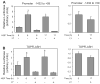
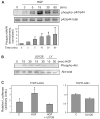
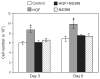
Hepatocyte growth factor (HGF) is upregulated in response to lung injury and has been implicated in tissue repair through its antiapoptotic and proliferative activities. Cyclooxygenase-2 (COX-2) is an inducible enzyme in the biosynthetic pathway of prostaglandins, and its activation has been shown to play a role in cell growth. Here, we report that HGF induces gene transcription of COX-2 in human bronchial epithelial cells (HBEpC). Treatment of HBEpC with HGF resulted in phosphorylation of the HGF receptor (c-Met), activation of Akt, and upregulation of COX-2 mRNA. Adenovirus-mediated gene transfer of a dominant negative (DN) Akt mutant revealed that HGF increased COX-2 mRNA in an Akt-dependent manner. COX-2 promoter analysis in luciferase reporter constructs showed that HGF regulation required the beta-catenin-responsive T cell factor-4 binding element (TBE). The HGF activation of the COX-2 gene transcription was blocked by DN mutant of beta-catenin or by inhibitors that blocked activation of Akt. Inhibition of p42/p44 MAPK pathway blocked HGF-mediated activation of beta-catenin gene transcription but not Akt activation, suggesting that p42/p44 MAPK acts in a parallel mechanism for beta-catenin activation. We also found that inhibition of COX-2 with NS-398 blocked HGF-induced growth in HBEpC. Together, the results show that the HGF increases COX-2 gene expression via an Akt-, MAPK-, and beta-catenin-dependent pathway in HBEpC.
Figures
Similar articles
-
Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells.J Cell Physiol. 2006 Jun;207(3):757-66. doi: 10.1002/jcp.20621. J Cell Physiol. 2006. PMID: 16508949
-
EV71 induces COX-2 expression via c-Src/PDGFR/PI3K/Akt/p42/p44 MAPK/AP-1 and NF-kappaB in rat brain astrocytes.J Cell Physiol. 2010 Aug;224(2):376-86. doi: 10.1002/jcp.22133. J Cell Physiol. 2010. PMID: 20333648
-
Signaling pathway involved in cyclooxygenase-2 up-regulation by hepatocyte growth factor in endometrial cancer cells.Oncol Rep. 2011 Oct;26(4):957-64. doi: 10.3892/or.2011.1348. Epub 2011 Jun 15. Oncol Rep. 2011. PMID: 21687953
-
Hepatocyte growth factor/scatter factor stimulates migration of rat mammary fibroblasts through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways.Eur J Biochem. 2001 Aug;268(16):4423-9. doi: 10.1046/j.1432-1327.2001.02363.x. Eur J Biochem. 2001. PMID: 11502202
-
MAP kinase and beta-catenin signaling in HGF induced RPE migration.Mol Vis. 2002 Dec 20;8:483-93. Mol Vis. 2002. PMID: 12500177
Cited by
-
A tandem repeat of a fragment of Listeria monocytogenes internalin B protein induces cell survival and proliferation.Am J Physiol Lung Cell Mol Physiol. 2010 Dec;299(6):L905-14. doi: 10.1152/ajplung.00094.2010. Epub 2010 Oct 1. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20889677 Free PMC article.
-
Lung epithelial-endothelial-mesenchymal signaling network with hepatocyte growth factor as a hub is involved in bronchopulmonary dysplasia.Front Cell Dev Biol. 2024 Sep 3;12:1462841. doi: 10.3389/fcell.2024.1462841. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39291265 Free PMC article. Review.
-
Hepatocyte growth factor inhibits apoptosis by the profibrotic factor angiotensin II via extracellular signal-regulated kinase 1/2 in endothelial cells and tissue explants.Mol Biol Cell. 2010 Dec;21(23):4240-50. doi: 10.1091/mbc.E10-04-0341. Epub 2010 Oct 6. Mol Biol Cell. 2010. PMID: 20926686 Free PMC article.
-
The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice.J Clin Invest. 2010 Jun;120(6):1950-60. doi: 10.1172/JCI38369. Epub 2010 May 24. J Clin Invest. 2010. PMID: 20501949 Free PMC article.
-
Characterization of the enhancing effect of protamine on the proliferative activity of hepatocyte growth factor in rat hepatocytes.Pharm Res. 2009 Apr;26(4):1012-21. doi: 10.1007/s11095-008-9810-1. Epub 2009 Jan 6. Pharm Res. 2009. PMID: 19127344
References
-
- Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003;63:728–734. - PubMed
-
- Bazan NG, Lukiw WJ. Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J Biol Chem. 2002;277:30359–30367. - PubMed
-
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Gen Dev. 2006;16:51–59. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- Chen JH, Wu CW, Kao HL, Chang HM, Li AF, Liu TY, Chi CW. Effects of COX-2 inhibitor on growth of human gastric cancer cells and its relation to hepatocyte growth factor. Cancer Lett. 2006;239:263–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

