Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers
- PMID: 18243434
- PMCID: PMC2288749
- DOI: 10.1016/j.vaccine.2007.12.024
Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers
Erratum in
- Vaccine. 2008 Jul 23;26(31):3946
Corrected and republished in
-
Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers.Vaccine. 2008 Jul 23;26(31):3947-57. doi: 10.1016/j.vaccine.2007.12.060. Vaccine. 2008. PMID: 18724414 Free PMC article. Clinical Trial.
Abstract
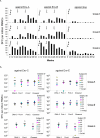
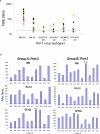
An optimally effective AIDS vaccine would likely require the induction of both neutralizing antibody and cell-mediated immune responses, which has proven difficult to obtain in previous clinical trials. Here we report on the induction of Human Immunodeficiency Virus Type-1 (HIV-1)-specific immune responses in healthy adult volunteers that received the multi-gene, polyvalent, DNA prime-protein boost HIV-1 vaccine formulation, DP6-001, in a Phase I clinical trial conducted in healthy adult volunteers of both genders. Robust cross-subtype HIV-1-specific T cell responses were detected in IFNgamma ELISPOT assays. Furthermore, we detected high titer serum antibody responses that recognized a wide range of primary HIV-1 Env antigens and also neutralized pseudotyped viruses that express the primary Env antigens from multiple HIV-1 subtypes. These findings demonstrate that the DNA prime-protein boost approach is an effective immunization method to elicit both humoral and cell-mediated immune responses in humans, and that a polyvalent Env formulation could generate broad immune responses against HIV-1 viruses with diverse genetic backgrounds.
Figures




Similar articles
-
Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers.Vaccine. 2008 Jul 23;26(31):3947-57. doi: 10.1016/j.vaccine.2007.12.060. Vaccine. 2008. PMID: 18724414 Free PMC article. Clinical Trial.
-
Fc Receptor-Mediated Activities of Env-Specific Human Monoclonal Antibodies Generated from Volunteers Receiving the DNA Prime-Protein Boost HIV Vaccine DP6-001.J Virol. 2016 Oct 28;90(22):10362-10378. doi: 10.1128/JVI.01458-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27630232 Free PMC article.
-
Screening of primary gp120 immunogens to formulate the next generation polyvalent DNA prime-protein boost HIV-1 vaccines.Hum Vaccin Immunother. 2017 Dec 2;13(12):2996-3009. doi: 10.1080/21645515.2017.1380137. Epub 2017 Sep 21. Hum Vaccin Immunother. 2017. PMID: 28933684 Free PMC article.
-
HIV-1 gp120 and Modified Vaccinia Virus Ankara (MVA) gp140 Boost Immunogens Increase Immunogenicity of a DNA/MVA HIV-1 Vaccine.J Virol. 2017 Nov 30;91(24):e01077-17. doi: 10.1128/JVI.01077-17. Print 2017 Dec 15. J Virol. 2017. PMID: 29021394 Free PMC article.
-
Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E.Virology. 2006 Jun 20;350(1):34-47. doi: 10.1016/j.virol.2006.02.032. Epub 2006 Apr 17. Virology. 2006. PMID: 16616287
Cited by
-
The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6-001) in healthy adult volunteers.Vaccine. 2008 Aug 18;26(35):4420-4. doi: 10.1016/j.vaccine.2008.05.090. Epub 2008 Jun 25. Vaccine. 2008. PMID: 18588934 Free PMC article. Clinical Trial.
-
Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits.Virology. 2009 Apr 25;387(1):147-56. doi: 10.1016/j.virol.2009.02.005. Epub 2009 Feb 27. Virology. 2009. PMID: 19249806 Free PMC article.
-
The influence of delivery vectors on HIV vaccine efficacy.Front Microbiol. 2014 Aug 22;5:439. doi: 10.3389/fmicb.2014.00439. eCollection 2014. Front Microbiol. 2014. PMID: 25202303 Free PMC article. Review.
-
A directed molecular evolution approach to improved immunogenicity of the HIV-1 envelope glycoprotein.PLoS One. 2011;6(6):e20927. doi: 10.1371/journal.pone.0020927. Epub 2011 Jun 29. PLoS One. 2011. PMID: 21738594 Free PMC article.
-
DNA priming and gp120 boosting induces HIV-specific antibodies in a randomized clinical trial.J Clin Invest. 2019 Nov 1;129(11):4769-4785. doi: 10.1172/JCI128699. J Clin Invest. 2019. PMID: 31566579 Free PMC article. Clinical Trial.
References
-
- HIV/AIDS JUNPo Report on the global AIDS epidemic 2006. 2006. [cited 2007 14 February]. Availablefrom: http://data.unaids.org/pub/GlobalReport/2006/2006_GR_CH02_en.pdf.
-
- Gorse GJ, Corey L, Patel GB, Mandava M, Hsieh RH, Matthews TJ, et al. HIV-1MN recombinant glycoprotein 160 vaccine-induced cellular and humoral immunity boosted by HIV-1MN recombinant glycoprotein 120 vaccine. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1999 Jan 20;15(2):115–32. - PubMed
-
- Kim JH, Pitisuttithum P, Kamboonruang C, Chuenchitra T, Mascola J, Frankel SS, et al. Specific antibody responses to vaccination with bivalent CM235/SF2 gp120: detection of homologous and heterologous neutralizing antibody to subtype E (CRF01.AE) HIV type 1. AIDS Res Hum Retroviruses. 2003 Sep;19(9):807–16. - PubMed
-
- Lee SA, Orque R, Escarpe PA, Peterson ML, Good JW, Zaharias EM, et al. Vaccine-induced antibodies to the native, oligomeric envelope glycoproteins of primary HIV-1 isolates. Vaccine. 2001 Nov 12;20(3−4):563–76. - PubMed
-
- Nitayaphan S, Khamboonruang C, Sirisophana N, Morgan P, Chiu J, Duliege AM, et al. A phase I/II trial of HIV SF2 gp120/MF59 vaccine in seronegative thais.AFRIMS-RIHES Vaccine Evaluation Group. Armed Forces Research Institute of Medical Sciences and the Research Institute for Health Sciences. Vaccine. 2000 Feb 14;18(15):1448–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI046294-02/AI/NIAID NIH HHS/United States
- AI46705/AI/NIAID NIH HHS/United States
- Z01 AI000883-07/ImNIH/Intramural NIH HHS/United States
- N01AI05394/AI/NIAID NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R01 AI065250-02/AI/NIAID NIH HHS/United States
- R29 AI040337-05/AI/NIAID NIH HHS/United States
- R21 AI046294/AI/NIAID NIH HHS/United States
- R01 AI065250/AI/NIAID NIH HHS/United States
- AI30034/AI/NIAID NIH HHS/United States
- 5P30DK32520/DK/NIDDK NIH HHS/United States
- R01AI65250/AI/NIAID NIH HHS/United States
- R29AI40337/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

