Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation
- PMID: 18239685
- PMCID: PMC2262037
- DOI: 10.1038/emboj.2008.5
Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation
Abstract
Nuclear factor kappa B (NF-kappaB) is a key mediator of inflammation. Unchecked NF-kappaB signalling can engender autoimmune pathologies and cancers. Here, we show that Tax1-binding protein 1 (TAX1BP1) is a negative regulator of TNF-alpha- and IL-1beta-induced NF-kappaB activation and that binding to mono- and polyubiquitin by a ubiquitin-binding Zn finger domain in TAX1BP1 is needed for TRAF6 association and NF-kappaB inhibition. Mice genetically knocked out for TAX1BP1 are born normal, but develop age-dependent inflammatory cardiac valvulitis, die prematurely, and are hypersensitive to low doses of TNF-alpha and IL-1beta. TAX1BP1-/- cells are more highly activated for NF-kappaB than control cells when stimulated with TNF-alpha or IL-1beta. Mechanistically, TAX1BP1 acts in NF-kappaB signalling as an essential adaptor between A20 and its targets.
Figures



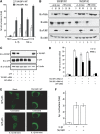
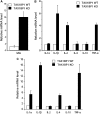



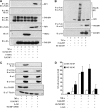
Similar articles
-
Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling.EMBO J. 2007 Sep 5;26(17):3910-22. doi: 10.1038/sj.emboj.7601823. Epub 2007 Aug 16. EMBO J. 2007. PMID: 17703191 Free PMC article.
-
The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1.Nat Immunol. 2011 Jul 17;12(9):834-43. doi: 10.1038/ni.2066. Nat Immunol. 2011. PMID: 21765415 Free PMC article.
-
Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes.Science. 2010 Feb 26;327(5969):1135-9. doi: 10.1126/science.1182364. Science. 2010. PMID: 20185725 Free PMC article.
-
TAX1BP1, a ubiquitin-binding adaptor protein in innate immunity and beyond.Trends Biochem Sci. 2011 Jul;36(7):347-54. doi: 10.1016/j.tibs.2011.03.004. Epub 2011 May 4. Trends Biochem Sci. 2011. PMID: 21546252 Review.
-
Cystic Fibrosis from Laboratory to Bedside: The Role of A20 in NF-κB-Mediated Inflammation.Med Princ Pract. 2015;24(4):301-10. doi: 10.1159/000381423. Epub 2015 Apr 25. Med Princ Pract. 2015. PMID: 25925366 Free PMC article. Review.
Cited by
-
Yeast one-hybrid screen of a thymus epithelial library identifies ZBTB7A as a regulator of thymic insulin expression.Mol Immunol. 2013 Dec;56(4):637-42. doi: 10.1016/j.molimm.2013.05.238. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911422 Free PMC article.
-
Altered expression of Tumor Necrosis Factor Alpha -Induced Protein 3 correlates with disease severity in Ulcerative Colitis.Sci Rep. 2017 Aug 25;7(1):9420. doi: 10.1038/s41598-017-09796-9. Sci Rep. 2017. PMID: 28842689 Free PMC article.
-
TAX1BP1 Restrains Virus-Induced Apoptosis by Facilitating Itch-Mediated Degradation of the Mitochondrial Adaptor MAVS.Mol Cell Biol. 2016 Dec 19;37(1):e00422-16. doi: 10.1128/MCB.00422-16. Print 2017 Jan 1. Mol Cell Biol. 2016. PMID: 27736772 Free PMC article.
-
An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport.Cell. 2016 Jun 30;166(1):152-66. doi: 10.1016/j.cell.2016.05.078. Cell. 2016. PMID: 27368102 Free PMC article.
-
Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation.EMBO J. 2012 Oct 3;31(19):3856-70. doi: 10.1038/emboj.2012.241. Epub 2012 Aug 28. EMBO J. 2012. PMID: 23032187 Free PMC article.
References
-
- Beg AA, Sha WC, Bronson RT, Baltimore D (1995) Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev 9: 2736–2746 - PubMed
-
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310: 1821–1824 - PubMed
-
- Boone DL (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 - PubMed
-
- Cao Z, Henzel WJ, Gao X (1996) IRAK: a kinase associated with the interleukin-1 receptor. Science 271: 1128–1131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

