Galectin-1 is a novel structural component and a major regulator of h-ras nanoclusters
- PMID: 18234837
- PMCID: PMC2291398
- DOI: 10.1091/mbc.e07-10-1053
Galectin-1 is a novel structural component and a major regulator of h-ras nanoclusters
Abstract
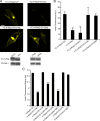
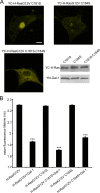
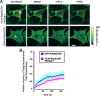
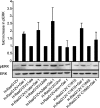
The organization of Ras proteins into nanoclusters on the inner plasma membrane is essential for Ras signal transduction, but the mechanisms that drive nanoclustering are unknown. Here we show that epidermal growth factor receptor activation stimulates the formation of H-Ras.GTP-Galectin-1 (Gal-1) complexes on the plasma membrane that are then assembled into transient nanoclusters. Gal-1 is therefore an integral structural component of the H-Ras-signaling nanocluster. Increasing Gal-1 levels increases the stability of H-Ras nanoclusters, leading to enhanced effector recruitment and signal output. Elements in the H-Ras C-terminal hypervariable region and an activated G-domain are required for H-Ras-Gal-1 interaction. Palmitoylation is not required for H-Ras-Gal-1 complex formation, but is required to anchor H-Ras-Gal-1 complexes to the plasma membrane. Our data suggest a mechanism for H-Ras nanoclustering that involves a dual role for Gal-1 as a critical scaffolding protein and a molecular chaperone that contributes to H-Ras trafficking by returning depalmitoylated H-Ras to the Golgi complex for repalmitoylation.
Figures
Similar articles
-
H-Ras nanocluster stability regulates the magnitude of MAPK signal output.PLoS One. 2010 Aug 5;5(8):e11991. doi: 10.1371/journal.pone.0011991. PLoS One. 2010. PMID: 20700538 Free PMC article.
-
Electrostatic interactions positively regulate K-Ras nanocluster formation and function.Mol Cell Biol. 2008 Jul;28(13):4377-85. doi: 10.1128/MCB.00050-08. Epub 2008 May 5. Mol Cell Biol. 2008. PMID: 18458061 Free PMC article.
-
Spatiotemporal organization of Ras signaling: rasosomes and the galectin switch.Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):471-95. doi: 10.1007/s10571-006-9059-3. Epub 2006 May 12. Cell Mol Neurobiol. 2006. PMID: 16691442 Free PMC article.
-
Ras nanoclusters: Versatile lipid-based signaling platforms.Biochim Biophys Acta. 2015 Apr;1853(4):841-9. doi: 10.1016/j.bbamcr.2014.09.008. Epub 2014 Sep 16. Biochim Biophys Acta. 2015. PMID: 25234412 Review.
-
Ras proteins: different signals from different locations.Nat Rev Mol Cell Biol. 2003 May;4(5):373-84. doi: 10.1038/nrm1105. Nat Rev Mol Cell Biol. 2003. PMID: 12728271 Review.
Cited by
-
Endomembrane H-Ras controls vascular endothelial growth factor-induced nitric-oxide synthase-mediated endothelial cell migration.J Biol Chem. 2013 May 24;288(21):15380-9. doi: 10.1074/jbc.M112.427765. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548900 Free PMC article.
-
N-Ras forms dimers at POPC membranes.Biophys J. 2012 Oct 3;103(7):1585-93. doi: 10.1016/j.bpj.2012.08.043. Epub 2012 Oct 2. Biophys J. 2012. PMID: 23062351 Free PMC article.
-
RAS Dimers: The Novice Couple at the RAS-ERK Pathway Ball.Genes (Basel). 2021 Sep 30;12(10):1556. doi: 10.3390/genes12101556. Genes (Basel). 2021. PMID: 34680951 Free PMC article. Review.
-
Protein localization: Can too much lipid glue stop Ras?Nat Chem Biol. 2010 Jul;6(7):483-5. doi: 10.1038/nchembio.399. Nat Chem Biol. 2010. PMID: 20559313 No abstract available.
-
The efficacy of Raf kinase recruitment to the GTPase H-ras depends on H-ras membrane conformer-specific nanoclustering.J Biol Chem. 2014 Apr 4;289(14):9519-33. doi: 10.1074/jbc.M113.537001. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24569991 Free PMC article.
References
-
- Ashery U., Yizhar O., Rotblat B., Elad-Sfadia G., Barkan B., Haklai R., Kloog Y. Spatiotemporal organization of Ras signaling: rasosomes and the galectin switch. Cell. Mol. Neurobiol. 2006;26:469–493. - PubMed
-
- Bivona T. G., et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell. 2006;21:481–493. - PubMed
-
- Bracha-Drori K., Shichrur K., Katz A., Oliva M., Angelovici R., Yalovsky S., Ohad N. Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J. 2004;40:419–427. - PubMed
-
- Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., 2nd, Cox A. D., Philips M. R. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 2002;4:343–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

