Osmotic water transport with glucose in GLUT2 and SGLT
- PMID: 18234816
- PMCID: PMC2367205
- DOI: 10.1529/biophysj.107.122531
Osmotic water transport with glucose in GLUT2 and SGLT
Abstract
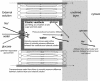
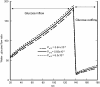
Carrier-mediated water cotransport is currently a favored explanation for water movement against an osmotic gradient. The vestibule within the central pore of Na(+)-dependent cotransporters or GLUT2 provides the necessary precondition for an osmotic mechanism, explaining this phenomenon without carriers. Simulating equilibrative glucose inflow via the narrow external orifice of GLUT2 raises vestibular tonicity relative to the external solution. Vestibular hypertonicity causes osmotic water inflow, which raises vestibular hydrostatic pressure and forces water, salt, and glucose into the outer cytosolic layer via its wide endofacial exit. Glucose uptake via GLUT2 also raises oocyte tonicity. Glucose exit from preloaded cells depletes the vestibule of glucose, making it hypotonic and thereby inducing water efflux. Inhibiting glucose exit with phloretin reestablishes vestibular hypertonicity, as it reequilibrates with the cytosolic glucose and net water inflow recommences. Simulated Na(+)-glucose cotransport demonstrates that active glucose accumulation within the vestibule generates water flows simultaneously with the onset of glucose flow and before any flow external to the transporter caused by hypertonicity in the outer cytosolic layers. The molar ratio of water/glucose flow is seen now to relate to the ratio of hydraulic and glucose permeability rather than to water storage capacity of putative water carriers.
Figures


Similar articles
-
Water transport by GLUT2 expressed in Xenopus laevis oocytes.J Physiol. 2007 Mar 1;579(Pt 2):345-61. doi: 10.1113/jphysiol.2006.123380. Epub 2006 Dec 7. J Physiol. 2007. PMID: 17158169 Free PMC article.
-
Glucose accumulation can account for the initial water flux triggered by Na+/glucose cotransport.Biophys J. 2004 Jan;86(1 Pt 1):125-33. doi: 10.1016/S0006-3495(04)74090-4. Biophys J. 2004. PMID: 14695256 Free PMC article.
-
Identification of new GLUT2-selective inhibitors through in silico ligand screening and validation in eukaryotic expression systems.Sci Rep. 2021 Jul 2;11(1):13751. doi: 10.1038/s41598-021-93063-5. Sci Rep. 2021. PMID: 34215797 Free PMC article.
-
SLC5 and SLC2 transporters in epithelia-cellular role and molecular mechanisms.Curr Top Membr. 2012;70:29-76. doi: 10.1016/B978-0-12-394316-3.00002-8. Curr Top Membr. 2012. PMID: 23177983 Review.
-
Coupling between Na+, sugar, and water transport across the intestine.Ann N Y Acad Sci. 2000;915:54-66. doi: 10.1111/j.1749-6632.2000.tb05223.x. Ann N Y Acad Sci. 2000. PMID: 11193601 Review.
Cited by
-
Membrane Phase-Dependent Occlusion of Intramolecular GLUT1 Cavities Demonstrated by Simulations.Biophys J. 2017 Mar 28;112(6):1176-1184. doi: 10.1016/j.bpj.2017.01.030. Biophys J. 2017. PMID: 28355545 Free PMC article.
-
Transient formation of water-conducting states in membrane transporters.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7696-701. doi: 10.1073/pnas.1218986110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610412 Free PMC article.
-
Does apical membrane GLUT2 have a role in intestinal glucose uptake?F1000Res. 2014 Dec 12;3:304. doi: 10.12688/f1000research.5934.1. eCollection 2014. F1000Res. 2014. PMID: 25671087 Free PMC article. Review.
-
Dietary L-arginine supplementation protects weanling pigs from deoxynivalenol-induced toxicity.Toxins (Basel). 2015 Apr 15;7(4):1341-54. doi: 10.3390/toxins7041341. Toxins (Basel). 2015. PMID: 25884909 Free PMC article.
-
Cotransport of water by the Na+-K+-2Cl(-) cotransporter NKCC1 in mammalian epithelial cells.J Physiol. 2010 Nov 1;588(Pt 21):4089-101. doi: 10.1113/jphysiol.2010.194738. J Physiol. 2010. PMID: 20819947 Free PMC article.
References
-
- Curran, P. F., and J. R. MacIntosh. 1962. A model system for biological water transport. Nature. 193:347–348. - PubMed
-
- Zeuthen, T., and W. D. Stein. 1994. Cotransport of salt and water in membrane proteins: membrane proteins as osmotic engines. J. Membr. Biol. 137:179–195. - PubMed
-
- Lapointe, J. Y. 2007. Response to Zeuthen and Zeuthen's comment to the editor: enough local hypertonicity is enough. Biophys. J. 93:1417–1419.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

