Adenovirus E1B55K region is required to enhance cyclin E expression for efficient viral DNA replication
- PMID: 18234796
- PMCID: PMC2268468
- DOI: 10.1128/JVI.01708-07
Adenovirus E1B55K region is required to enhance cyclin E expression for efficient viral DNA replication
Abstract
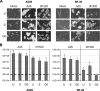
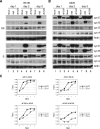
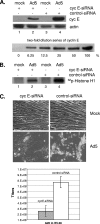
Adenoviruses (Ads) with E1B55K mutations can selectively replicate in and destroy cancer cells. However, the mechanism of Ad-selective replication in tumor cells is not well characterized. We have shown previously that expression of several cell cycle-regulating genes is markedly affected by the Ad E1b gene in WI-38 human lung fibroblast cells (X. Rao, et al., Virology 350:418-428, 2006). In the current study, we show that the Ad E1B55K region is required to enhance cyclin E expression and that the failure to induce cyclin E overexpression due to E1B55K mutations prevents viral DNA from undergoing efficient replication in WI-38 cells, especially when the cells are arrested in the G(0) phase of the cell cycle by serum starvation. In contrast, cyclin E induction is less dependent on the function encoded in the E1B55K region in A549 and other cancer cells that are permissive for replication of E1B55K-mutated viruses, whether the cells are in the S phase or G(0) phase. The small interfering RNA that specifically inhibits cyclin E expression partially decreased viral replication. Our study provides evidence suggesting that E1B55K may be involved in cell cycle regulation that is important for efficient viral DNA replication and that cyclin E overexpression in cancer cells may be associated with the oncolytic replication of E1B55K-mutated viruses.
Figures
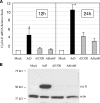
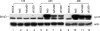
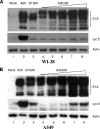

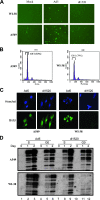

Similar articles
-
Oncolytic Replication of E1b-Deleted Adenoviruses.Viruses. 2015 Nov 6;7(11):5767-79. doi: 10.3390/v7112905. Viruses. 2015. PMID: 26561828 Free PMC article. Review.
-
Molecular basis for viral selective replication in cancer cells: activation of CDK2 by adenovirus-induced cyclin E.PLoS One. 2013;8(2):e57340. doi: 10.1371/journal.pone.0057340. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437375 Free PMC article.
-
Selective replication of E1B55K-deleted adenoviruses depends on enhanced E1A expression in cancer cells.Cancer Gene Ther. 2006 Jun;13(6):572-83. doi: 10.1038/sj.cgt.7700923. Cancer Gene Ther. 2006. PMID: 16341141
-
Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts.J Virol. 2006 Jan;80(2):964-74. doi: 10.1128/JVI.80.2.964-974.2006. J Virol. 2006. PMID: 16378998 Free PMC article.
-
[Development of Novel Genetically Engineered Adenoviruses Based on Functional Analyses of Adenovirus-encoded Small RNAs].Yakugaku Zasshi. 2016;136(11):1509-1515. doi: 10.1248/yakushi.16-00170. Yakugaku Zasshi. 2016. PMID: 27803482 Review. Japanese.
Cited by
-
The role of JNK phosphorylation as a molecular target to enhance adenovirus replication, oncolysis and cancer therapeutic efficacy.Cancer Biol Ther. 2018;19(12):1174-1184. doi: 10.1080/15384047.2018.1491503. Epub 2018 Aug 1. Cancer Biol Ther. 2018. PMID: 30067431 Free PMC article.
-
Targeting Palbociclib-Resistant Estrogen Receptor-Positive Breast Cancer Cells via Oncolytic Virotherapy.Cancers (Basel). 2019 May 16;11(5):684. doi: 10.3390/cancers11050684. Cancers (Basel). 2019. PMID: 31100952 Free PMC article.
-
Indole-3-carbinol (I3C) increases apoptosis, represses growth of cancer cells, and enhances adenovirus-mediated oncolysis.Cancer Biol Ther. 2014 Sep;15(9):1256-67. doi: 10.4161/cbt.29690. Epub 2014 Jun 27. Cancer Biol Ther. 2014. PMID: 24972095 Free PMC article.
-
Enhanced oncolytic adenoviral production by downregulation of death-domain associated protein and overexpression of precursor terminal protein.Sci Rep. 2021 Jan 13;11(1):856. doi: 10.1038/s41598-020-79998-1. Sci Rep. 2021. PMID: 33441685 Free PMC article.
-
Gambogenic acid-induced time- and dose-dependent growth inhibition and apoptosis involving Akt pathway inactivation in U251 glioblastoma cells.J Nat Med. 2012 Jan;66(1):62-9. doi: 10.1007/s11418-011-0553-7. Epub 2011 Aug 31. J Nat Med. 2012. PMID: 21879332
References
-
- Bagchi, S., P. Raychaudhuri, and J. R. Nevins. 1990. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell 62659-669. - PubMed
-
- Bandara, L. R., and N. B. La Thangue. 1991. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature 351494-497. - PubMed
-
- Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 156107-121. - PubMed
-
- Berglund, P., and G. Landberg. 2006. Cyclin e overexpression reduces infiltrative growth in breast cancer: yet another link between proliferation control and tumor invasion. Cell Cycle 5606-609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

