The PINK1/Parkin pathway regulates mitochondrial morphology
- PMID: 18230723
- PMCID: PMC2234197
- DOI: 10.1073/pnas.0709336105
The PINK1/Parkin pathway regulates mitochondrial morphology
Abstract
Loss-of-function mutations in the PTEN-induced kinase 1 (PINK1) or parkin genes, which encode a mitochondrially localized serine/threonine kinase and a ubiquitin-protein ligase, respectively, result in recessive familial forms of Parkinsonism. Genetic studies in Drosophila indicate that PINK1 acts upstream of Parkin in a common pathway that influences mitochondrial integrity in a subset of tissues, including flight muscle and dopaminergic neurons. The mechanism by which PINK1 and Parkin influence mitochondrial integrity is currently unknown, although mutations in the PINK1 and parkin genes result in enlarged or swollen mitochondria, suggesting a possible regulatory role for the PINK1/Parkin pathway in mitochondrial morphology. To address this hypothesis, we examined the influence of genetic alterations affecting the machinery that governs mitochondrial morphology on the PINK1 and parkin mutant phenotypes. We report that heterozygous loss-of-function mutations of drp1, which encodes a key mitochondrial fission-promoting component, are largely lethal in a PINK1 or parkin mutant background. Conversely, the flight muscle degeneration and mitochondrial morphological alterations that result from mutations in PINK1 and parkin are strongly suppressed by increased drp1 gene dosage and by heterozygous loss-of-function mutations affecting the mitochondrial fusion-promoting factors OPA1 and Mfn2. Finally, we find that an eye phenotype associated with increased PINK1/Parkin pathway activity is suppressed by perturbations that reduce mitochondrial fission and enhanced by perturbations that reduce mitochondrial fusion. Our studies suggest that the PINK1/Parkin pathway promotes mitochondrial fission and that the loss of mitochondrial and tissue integrity in PINK1 and parkin mutants derives from reduced mitochondrial fission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
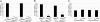
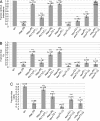
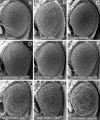

Similar articles
-
The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14503-8. doi: 10.1073/pnas.0803998105. Epub 2008 Sep 17. Proc Natl Acad Sci U S A. 2008. PMID: 18799731 Free PMC article.
-
The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process.Biochem Biophys Res Commun. 2009 Jan 16;378(3):518-23. doi: 10.1016/j.bbrc.2008.11.086. Epub 2008 Dec 3. Biochem Biophys Res Commun. 2009. PMID: 19056353
-
Atg1-mediated autophagy suppresses tissue degeneration in pink1/parkin mutants by promoting mitochondrial fission in Drosophila.Mol Biol Cell. 2018 Dec 15;29(26):3082-3092. doi: 10.1091/mbc.E18-04-0243. Epub 2018 Oct 24. Mol Biol Cell. 2018. PMID: 30354903 Free PMC article.
-
The PINK1/Parkin pathway: a mitochondrial quality control system?J Bioenerg Biomembr. 2009 Dec;41(6):499-503. doi: 10.1007/s10863-009-9253-3. J Bioenerg Biomembr. 2009. PMID: 19967438 Review.
-
PINK1-Parkin signaling in Parkinson's disease: Lessons from Drosophila.Neurosci Res. 2020 Oct;159:40-46. doi: 10.1016/j.neures.2020.01.016. Epub 2020 Feb 6. Neurosci Res. 2020. PMID: 32035987 Review.
Cited by
-
Prematurity alters the progenitor cell program of the upper respiratory tract of neonates.Sci Rep. 2021 May 24;11(1):10799. doi: 10.1038/s41598-021-90093-x. Sci Rep. 2021. PMID: 34031475 Free PMC article.
-
The role of PINK1-Parkin in mitochondrial quality control.Nat Cell Biol. 2024 Oct;26(10):1639-1651. doi: 10.1038/s41556-024-01513-9. Epub 2024 Oct 2. Nat Cell Biol. 2024. PMID: 39358449 Review.
-
Mouse models of Parkinson's disease associated with mitochondrial dysfunction.Mol Cell Neurosci. 2013 Jul;55:87-94. doi: 10.1016/j.mcn.2012.08.002. Epub 2012 Aug 11. Mol Cell Neurosci. 2013. PMID: 22954895 Free PMC article. Review.
-
Mitochondrial dynamics: the intersection of form and function.Adv Exp Med Biol. 2012;748:13-40. doi: 10.1007/978-1-4614-3573-0_2. Adv Exp Med Biol. 2012. PMID: 22729853 Free PMC article. Review.
-
Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences.EMBO J. 2012 Jun 26;31(14):3038-62. doi: 10.1038/emboj.2012.170. EMBO J. 2012. PMID: 22735187 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

