Agonist binding, agonist affinity and agonist efficacy at G protein-coupled receptors
- PMID: 18223670
- PMCID: PMC2437915
- DOI: 10.1038/sj.bjp.0707672
Agonist binding, agonist affinity and agonist efficacy at G protein-coupled receptors
Abstract
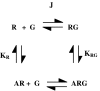
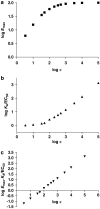
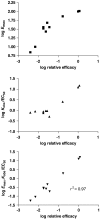
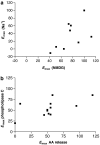
Measurements of affinity and efficacy are fundamental for work on agonists both in drug discovery and in basic studies on receptors. In this review I wish to consider methods for measuring affinity and efficacy at G protein coupled receptors (GPCRs). Agonist affinity may be estimated in terms of the dissociation constant for agonist binding to a receptor using ligand binding or functional assays. It has, however, been suggested that measurements of affinity are always contaminated by efficacy so that it is impossible to separate the two parameters. Here I show that for many GPCRs, if receptor/G protein coupling is suppressed, experimental measurements of agonist affinity using ligand binding (K(obs)) provide quite accurate measures of the agonist microscopic dissociation constant (KA). Also in pharmacological functional studies, good estimates of agonist dissociation constants are possible. Efficacy can be quantitated in several ways based on functional data (maximal effect of the agonist (E(max)), ratio of agonist dissociation constant to concentration of agonist giving half maximal effect in functional assay (K(obs)/EC50), a combined parameter E(max)K(obs)/EC50). Here I show that E(max)K(obs)/EC50 provides the best assessment of efficacy for a range of agonists across the full range of efficacy for full to partial agonists. Considerable evidence now suggests that ligand efficacy may be dependent on the pathway used to assess it. The efficacy of a ligand may, therefore, be multidimensional. It is still, however, necessary to have accurate measures of efficacy in different pathways.
Figures
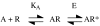
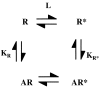
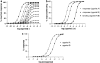
Similar articles
-
Estimation of the receptor-state affinity constants of ligands in functional studies using wild type and constitutively active mutant receptors: Implications for estimation of agonist bias.J Pharmacol Toxicol Methods. 2017 Jan-Feb;83:94-106. doi: 10.1016/j.vascn.2016.09.007. Epub 2016 Oct 7. J Pharmacol Toxicol Methods. 2017. PMID: 27725245 Free PMC article.
-
Quantifying agonist activity at G protein-coupled receptors.J Vis Exp. 2011 Dec 26;(58):e3179. doi: 10.3791/3179. J Vis Exp. 2011. PMID: 22231636 Free PMC article.
-
Quantifying biological activity in chemical terms: a pharmacology primer to describe drug effect.ACS Chem Biol. 2009 Apr 17;4(4):249-60. doi: 10.1021/cb800299s. Epub 2009 Feb 4. ACS Chem Biol. 2009. PMID: 19193052
-
Unravelling intrinsic efficacy and ligand bias at G protein coupled receptors: A practical guide to assessing functional data.Biochem Pharmacol. 2016 Feb 1;101:1-12. doi: 10.1016/j.bcp.2015.10.011. Epub 2015 Oct 23. Biochem Pharmacol. 2016. PMID: 26478533 Review.
-
Mechanisms underlying agonist efficacy.Biochem Soc Trans. 2007 Aug;35(Pt 4):733-6. doi: 10.1042/BST0350733. Biochem Soc Trans. 2007. PMID: 17635136 Review.
Cited by
-
Identification of essential residues for binding and activation in the human 5-HT7(a) serotonin receptor by molecular modeling and site-directed mutagenesis.Front Behav Neurosci. 2015 May 8;9:92. doi: 10.3389/fnbeh.2015.00092. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26005408 Free PMC article.
-
Use of the GTPγS ([35S]GTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors.Br J Pharmacol. 2010 Nov;161(6):1238-49. doi: 10.1111/j.1476-5381.2010.00963.x. Br J Pharmacol. 2010. PMID: 20662841 Free PMC article. Review.
-
Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter.J Pharmacol Exp Ther. 2023 Apr;385(1):62-75. doi: 10.1124/jpet.122.001454. Epub 2023 Jan 20. J Pharmacol Exp Ther. 2023. PMID: 36669875 Free PMC article.
-
An estimation of beta 2-adrenoceptor reserve on human bronchial smooth muscle for some sympathomimetic bronchodilators.Br J Pharmacol. 2009 Sep;158(1):287-99. doi: 10.1111/j.1476-5381.2009.00277.x. Epub 2009 May 14. Br J Pharmacol. 2009. PMID: 19466988 Free PMC article.
-
Misfolding Ectodomain Mutations of the Lutropin Receptor Increase Efficacy of Hormone Stimulation.Mol Endocrinol. 2016 Jan;30(1):62-76. doi: 10.1210/me.2015-1205. Epub 2015 Nov 10. Mol Endocrinol. 2016. PMID: 26554443 Free PMC article.
References
-
- Avalos M, Mak C, Randall PK, Trzeciakowski JP, Abell C, Kwan SW, et al. Nonlinear analysis of partial dopamine agonist effects on cAMP in C6 glioma cells. J Pharmacol Toxicol Methods. 2001;45:17–37. - PubMed
-
- Besse JC, Furchgott RF. Dissociation constants and relative efficacies of agonists acting on alpha adrenergic receptors in rabbit aorta. J Pharmacol Exp Ther. 1976;197:66–78. - PubMed
-
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc London B Biol Sci. 1983;220:141–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

