Small-molecule aggregates inhibit amyloid polymerization
- PMID: 18223646
- PMCID: PMC2730835
- DOI: 10.1038/nchembio.65
Small-molecule aggregates inhibit amyloid polymerization
Abstract
Many amyloid inhibitors resemble molecules that form chemical aggregates, which are known to inhibit many proteins. Eight known chemical aggregators inhibited amyloid formation of the yeast and mouse prion proteins Sup35 and recMoPrP in a manner characteristic of colloidal inhibition. Similarly, three known anti-amyloid molecules inhibited beta-lactamase in a detergent-dependent manner, which suggests that they too form colloidal aggregates. The colloids localized to preformed fibers and prevented new fiber formation in electron micrographs. They also blocked infection of yeast cells with Sup35 prions, which suggests that colloidal inhibition may be relevant in more biological milieus.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures
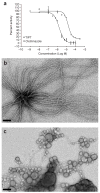
Comment in
-
Aggregator compounds confound amyloid fibrillization assay.Nat Chem Biol. 2008 Mar;4(3):159-60. doi: 10.1038/nchembio0308-159. Nat Chem Biol. 2008. PMID: 18277973 No abstract available.
Similar articles
-
Aggregator compounds confound amyloid fibrillization assay.Nat Chem Biol. 2008 Mar;4(3):159-60. doi: 10.1038/nchembio0308-159. Nat Chem Biol. 2008. PMID: 18277973 No abstract available.
-
Countering amyloid polymorphism and drug resistance with minimal drug cocktails.Prion. 2010 Oct-Dec;4(4):244-51. doi: 10.4161/pri.4.4.13597. Epub 2010 Oct 12. Prion. 2010. PMID: 20935457 Free PMC article.
-
A synergistic small-molecule combination directly eradicates diverse prion strain structures.Nat Chem Biol. 2009 Dec;5(12):936-46. doi: 10.1038/nchembio.246. Epub 2009 Nov 1. Nat Chem Biol. 2009. PMID: 19915541 Free PMC article.
-
[Mechanism and application of molecular self-assembly in Sup35 prion domain of Saccharomyces cerevisiae].Sheng Wu Gong Cheng Xue Bao. 2011 Oct;27(10):1401-7. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 22260056 Review. Chinese.
-
[New aspects of research upon the yeast Saccharomyces cerevisiae [PSI+] prion].Postepy Biochem. 2007;53(2):182-7. Postepy Biochem. 2007. PMID: 17969880 Review. Polish.
Cited by
-
Comparison of three amyloid assembly inhibitors: the sugar scyllo-inositol, the polyphenol epigallocatechin gallate, and the molecular tweezer CLR01.ACS Chem Neurosci. 2012 Jun 20;3(6):451-8. doi: 10.1021/cn200133x. Epub 2012 Mar 7. ACS Chem Neurosci. 2012. PMID: 22860214 Free PMC article.
-
Natural compounds may open new routes to treatment of amyloid diseases.Neurotherapeutics. 2013 Jul;10(3):429-39. doi: 10.1007/s13311-013-0192-7. Neurotherapeutics. 2013. PMID: 23670234 Free PMC article. Review.
-
Fighting nature with nature: antiviral compounds that target retroviruses.Arch Microbiol. 2024 Feb 28;206(3):130. doi: 10.1007/s00203-024-03846-3. Arch Microbiol. 2024. PMID: 38416180 Review.
-
A simple in vitro assay for assessing the efficacy, mechanisms and kinetics of anti-prion fibril compounds.Prion. 2018;12(5-6):280-300. doi: 10.1080/19336896.2018.1525254. Epub 2018 Oct 9. Prion. 2018. PMID: 30223704 Free PMC article.
-
Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies.Nat Rev Drug Discov. 2009 Oct;8(10):783-93. doi: 10.1038/nrd2959. Nat Rev Drug Discov. 2009. PMID: 19794442 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/46499856
- PubChem-Substance/46499857
- PubChem-Substance/46499858
- PubChem-Substance/46499859
- PubChem-Substance/46499860
- PubChem-Substance/46499861
- PubChem-Substance/46499862
- PubChem-Substance/46499863
- PubChem-Substance/46499864
- PubChem-Substance/46499865
- PubChem-Substance/46499866
- PubChem-Substance/46499867
- PubChem-Substance/46499868
- PubChem-Substance/46499869
- PubChem-Substance/46499870
- PubChem-Substance/46499871
- PubChem-Substance/46499872
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

