In vitro activation of the IkappaB kinase complex by human T-cell leukemia virus type-1 Tax
- PMID: 18223255
- PMCID: PMC2397464
- DOI: 10.1074/jbc.M704831200
In vitro activation of the IkappaB kinase complex by human T-cell leukemia virus type-1 Tax
Abstract
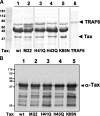
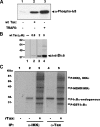
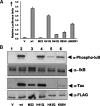
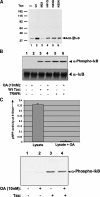
Human T-cell leukemia virus type-I expresses Tax, a 40-kDa oncoprotein that activates IkappaB kinase (IKK), resulting in constitutive activation of NFkappaB. Herein, we have developed an in vitro signaling assay to analyze IKK complex activation by recombinant Tax. Using this assay in combination with reporter assays, we demonstrate that Tax-mediated activation of IKK is independent of phosphatases. We show that sustained activation of the Tax-mediated activation of the NFkappaB pathway is dependent on an intact Hsp90-IKK complex. By acetylating and thereby preventing activation of the IKK complex by the Yersinia effector YopJ, we demonstrate that Tax-mediated activation of the IKK complex requires a phosphorylation step. Our characterization of an in vitro signaling assay system for the mechanism of Tax-mediated activation of the IKK complex with a variety of mutants and inhibitors results in a working model for the biochemical mechanism of Tax-induced activation.
Figures

Similar articles
-
Activation of the IκB kinase complex by HTLV-1 Tax requires cytosolic factors involved in Tax-induced polyubiquitination.J Biochem. 2011 Dec;150(6):679-86. doi: 10.1093/jb/mvr106. Epub 2011 Aug 23. J Biochem. 2011. PMID: 21862596
-
An alternative splice product of IkappaB kinase (IKKgamma), IKKgamma-delta, differentially mediates cytokine and human T-cell leukemia virus type 1 tax-induced NF-kappaB activation.J Virol. 2006 May;80(9):4227-41. doi: 10.1128/JVI.80.9.4227-4241.2006. J Virol. 2006. PMID: 16611882 Free PMC article.
-
Activation of NF-kappa B by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of I kappa B kinase.J Biol Chem. 2007 Feb 9;282(6):4185-92. doi: 10.1074/jbc.M611031200. Epub 2006 Dec 4. J Biol Chem. 2007. PMID: 17145747
-
Activation of I-kappaB kinase by the HTLV type 1 Tax protein: mechanistic insights into the adaptor function of IKKgamma.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1591-6. doi: 10.1089/08892220050193001. AIDS Res Hum Retroviruses. 2000. PMID: 11080796 Review.
-
Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B.Cytokine Growth Factor Rev. 2001 Jun-Sep;12(2-3):207-17. doi: 10.1016/s1359-6101(00)00028-9. Cytokine Growth Factor Rev. 2001. PMID: 11325603 Review.
Cited by
-
How to rewire the host cell: A home improvement guide for intracellular bacteria.J Cell Biol. 2017 Dec 4;216(12):3931-3948. doi: 10.1083/jcb.201701095. Epub 2017 Nov 2. J Cell Biol. 2017. PMID: 29097627 Free PMC article. Review.
-
HTLV-1 and apoptosis: role in cellular transformation and recent advances in therapeutic approaches.Apoptosis. 2008 Jun;13(6):733-47. doi: 10.1007/s10495-008-0208-7. Apoptosis. 2008. PMID: 18421579 Free PMC article. Review.
-
The modulation of apoptosis by oncogenic viruses.Virol J. 2013 Jun 6;10:182. doi: 10.1186/1743-422X-10-182. Virol J. 2013. PMID: 23741982 Free PMC article. Review.
-
Tipping the balance by manipulating post-translational modifications.Curr Opin Microbiol. 2010 Feb;13(1):34-40. doi: 10.1016/j.mib.2009.12.004. Epub 2010 Jan 12. Curr Opin Microbiol. 2010. PMID: 20071215 Free PMC article. Review.
-
Anti-apoptotic effect of Tax: an NF-κB path or a CREB way?Viruses. 2011 Jul;3(7):1001-14. doi: 10.3390/v3071001. Epub 2011 Jun 27. Viruses. 2011. PMID: 21994767 Free PMC article. Review.
References
-
- Yoshida, M. (2001) Annu. Rev. Immunol. 19 475-496 - PubMed
-
- Chu, Z. L., Shin, Y. A., Yang, J. M., DiDonato, J. A., and Ballard, D. W. (1999) J. Biol. Chem. 274 15297-15300 - PubMed
-
- Jin, D. Y., Giordano, V., Kibler, K. V., Nakano, H., and Jeang, K. T. (1999) J. Biol. Chem. 274 17402-17405 - PubMed
-
- Xiao, G., Harhaj, E. W., and Sun, S. C. (2000) J. Biol. Chem. 275 34060-34067 - PubMed
-
- Harhaj, E. W., and Harhaj, N. S. (2005) IUBMB Life 57 83-91 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

