Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory
- PMID: 18223147
- PMCID: PMC2259048
- DOI: 10.1104/pp.107.115691
Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory
Abstract
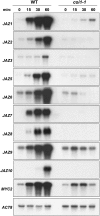
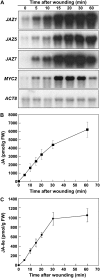
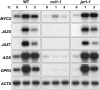
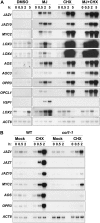
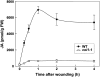
Jasmonate (JA) and its amino acid conjugate, jasmonoyl-isoleucine (JA-Ile), play important roles in regulating plant defense responses to insect herbivores. Recent studies indicate that JA-Ile promotes the degradation of JASMONATE ZIM-domain (JAZ) transcriptional repressors through the activity of the E(3) ubiquitin-ligase SCF(COI1). Here, we investigated the regulation and function of JAZ genes during the interaction of Arabidopsis (Arabidopsis thaliana) with the generalist herbivore Spodoptera exigua. Most members of the JAZ gene family were highly expressed in response to S. exigua feeding and mechanical wounding. JAZ transcript levels increased within 5 min of mechanical tissue damage, coincident with a large (approximately 25-fold) rise in JA and JA-Ile levels. Wound-induced expression of JAZ and other CORONATINE-INSENSITIVE1 (COI1)-dependent genes was not impaired in the jar1-1 mutant that is partially deficient in the conversion of JA to JA-Ile. Experiments performed with the protein synthesis inhibitor cycloheximide provided evidence that JAZs, MYC2, and genes encoding several JA biosynthetic enzymes are primary response genes whose expression is derepressed upon COI1-dependent turnover of a labile repressor protein(s). We also show that overexpression of a modified form of JAZ1 (JAZ1Delta3A) that is stable in the presence of JA compromises host resistance to feeding by S. exigua larvae. These findings establish a role for JAZ proteins in the regulation of plant anti-insect defense, and support the hypothesis that JA-Ile and perhaps other JA derivatives activate COI1-dependent wound responses in Arabidopsis. Our results also indicate that the timing of JA-induced transcription in response to wounding is more rapid than previously realized.
Figures


Similar articles
-
A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein.Plant J. 2008 Sep;55(6):979-88. doi: 10.1111/j.1365-313X.2008.03566.x. Epub 2008 Jun 10. Plant J. 2008. PMID: 18547396 Free PMC article.
-
Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin.Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):E8930-E8939. doi: 10.1073/pnas.1710885114. Epub 2017 Oct 2. Proc Natl Acad Sci U S A. 2017. PMID: 28973940 Free PMC article.
-
JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling.Nature. 2007 Aug 9;448(7154):661-5. doi: 10.1038/nature05960. Epub 2007 Jul 18. Nature. 2007. PMID: 17637677
-
JAZ repressors set the rhythm in jasmonate signaling.Curr Opin Plant Biol. 2008 Oct;11(5):486-94. doi: 10.1016/j.pbi.2008.06.003. Epub 2008 Jul 22. Curr Opin Plant Biol. 2008. PMID: 18653378 Review.
-
Arabidopsis jasmonate signaling pathway.Sci Signal. 2010 Feb 16;3(109):cm4. doi: 10.1126/scisignal.3109cm4. Sci Signal. 2010. PMID: 20159850 Review.
Cited by
-
The C2 Protein from the Geminivirus Tomato Yellow Leaf Curl Sardinia Virus Decreases Sensitivity to Jasmonates and Suppresses Jasmonate-Mediated Defences.Plants (Basel). 2016 Jan 15;5(1):8. doi: 10.3390/plants5010008. Plants (Basel). 2016. PMID: 27135228 Free PMC article.
-
Jasmonates and Plant Salt Stress: Molecular Players, Physiological Effects, and Improving Tolerance by Using Genome-Associated Tools.Int J Mol Sci. 2021 Mar 17;22(6):3082. doi: 10.3390/ijms22063082. Int J Mol Sci. 2021. PMID: 33802953 Free PMC article. Review.
-
Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany.Ann Bot. 2013 Jun;111(6):1021-58. doi: 10.1093/aob/mct067. Epub 2013 Apr 4. Ann Bot. 2013. PMID: 23558912 Free PMC article. Review.
-
Transcriptional analysis of distant signaling induced by insect elicitors and mechanical wounding in Zea mays.PLoS One. 2012;7(4):e34855. doi: 10.1371/journal.pone.0034855. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511969 Free PMC article.
-
Jasmonates: Multifunctional Roles in Stress Tolerance.Front Plant Sci. 2016 Jun 15;7:813. doi: 10.3389/fpls.2016.00813. eCollection 2016. Front Plant Sci. 2016. PMID: 27379115 Free PMC article. Review.
References
-
- Abel S (2007) Auxin is surfacing. ACS Chem Biol 2 380–384 - PubMed
-
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251 533–549 - PubMed
-
- Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177 301–318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

