ESCRT complexes and the biogenesis of multivesicular bodies
- PMID: 18222686
- PMCID: PMC2282067
- DOI: 10.1016/j.ceb.2007.12.002
ESCRT complexes and the biogenesis of multivesicular bodies
Abstract
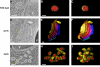
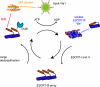
Multivesicular bodies (MVBs) are crucial intermediates in the trafficking of ubiquitinated receptors and other cargo destined for lysosomes. The formation of MVBs by invagination of the endosomal limiting membrane is catalyzed by the endosomal sorting complex required for transport (ESCRT) complexes, a process that has recently been visualized in three-dimensional detail by electron tomography. Structural and biochemical analysis of the upstream components, Vps27-Hse1, ESCRT-I, and ESCRT-II, shows how these complexes assemble and cluster cargo. Rapid progress has been made in understanding the assembly and disassembly of the ESCRT-III complex and the interactions of its subunits with MIT domain and other proteins. A key role for deubiquitination in the regulation of the system has been demonstrated. One central question remains largely unanswered, which is how the ESCRTs actually promote the invagination of the endosomal membrane.
Figures


Similar articles
-
Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome.J Cell Biol. 2003 Oct 27;163(2):237-43. doi: 10.1083/jcb.200305007. J Cell Biol. 2003. PMID: 14581452 Free PMC article.
-
Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation.Dev Cell. 2008 Oct;15(4):578-89. doi: 10.1016/j.devcel.2008.08.013. Dev Cell. 2008. PMID: 18854142
-
Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes.J Cell Biol. 2006 Dec 4;175(5):715-20. doi: 10.1083/jcb.200606113. Epub 2006 Nov 27. J Cell Biol. 2006. PMID: 17130288 Free PMC article.
-
Protein sorting into multivesicular endosomes.Curr Opin Cell Biol. 2003 Aug;15(4):446-55. doi: 10.1016/s0955-0674(03)00080-2. Curr Opin Cell Biol. 2003. PMID: 12892785 Review.
-
A concentric circle model of multivesicular body cargo sorting.EMBO Rep. 2007 Jul;8(7):644-50. doi: 10.1038/sj.embor.7401004. EMBO Rep. 2007. PMID: 17603537 Free PMC article. Review.
Cited by
-
Lysosomal fusion dysfunction as a unifying hypothesis for Alzheimer's disease pathology.Int J Alzheimers Dis. 2012;2012:752894. doi: 10.1155/2012/752894. Epub 2012 Aug 30. Int J Alzheimers Dis. 2012. PMID: 22970406 Free PMC article.
-
Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2022 Sep 20;23(19):11047. doi: 10.3390/ijms231911047. Int J Mol Sci. 2022. PMID: 36232350 Free PMC article. Review.
-
The ubiquitin ligases Cbl and Cbl-b regulate macrophage growth by controlling CSF-1R import into macropinosomes.Mol Biol Cell. 2024 Mar 1;35(3):ar38. doi: 10.1091/mbc.E23-09-0345. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170572 Free PMC article.
-
Coordination of substrate binding and ATP hydrolysis in Vps4-mediated ESCRT-III disassembly.Mol Biol Cell. 2010 Oct 1;21(19):3396-408. doi: 10.1091/mbc.E10-06-0512. Epub 2010 Aug 11. Mol Biol Cell. 2010. PMID: 20702581 Free PMC article.
-
Tsg101/ESCRT-I recruitment regulated by the dual binding modes of K63-linked diubiquitin.Structure. 2022 Feb 3;30(2):289-299.e6. doi: 10.1016/j.str.2021.09.006. Structure. 2022. PMID: 35120596 Free PMC article.
References
-
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. - PubMed
-
- Russell MRG, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr. Opin. Cell Biol. 2006;18:422–428. - PubMed
-
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. - PubMed
-
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. ESCRT-III: An endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 2002;3:271–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

