Ectromelia virus BTB/kelch proteins, EVM150 and EVM167, interact with cullin-3-based ubiquitin ligases
- PMID: 18221766
- PMCID: PMC2441824
- DOI: 10.1016/j.virol.2007.11.036
Ectromelia virus BTB/kelch proteins, EVM150 and EVM167, interact with cullin-3-based ubiquitin ligases
Abstract
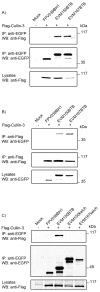
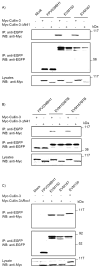
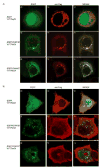
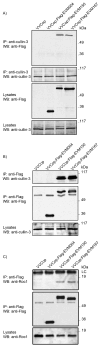
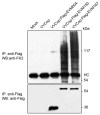
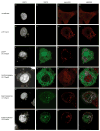
Cellular proteins containing BTB and kelch domains have been shown to function as adapters for the recruitment of substrates to cullin-3-based ubiquitin ligases. Poxviruses are the only family of viruses known to encode multiple BTB/kelch proteins, suggesting that poxviruses may modulate the ubiquitin pathway through interaction with cullin-3. Ectromelia virus encodes four BTB/kelch proteins and one BTB-only protein. Here we demonstrate that two of the ectromelia virus-encoded BTB/kelch proteins, EVM150 and EVM167, interacted with cullin-3. Similar to cellular BTB proteins, the BTB domain of EVM150 and EVM167 was necessary and sufficient for cullin-3 interaction. During infection, EVM150 and EVM167 localized to discrete cytoplasmic regions, which co-localized with cullin-3. Furthermore, EVM150 and EVM167 co-localized and interacted with conjugated ubiquitin, as demonstrated by confocal microscopy and co-immunoprecipitation. Our findings suggest that the ectromelia virus-encoded BTB/kelch proteins, EVM150 and EVM167, interact with cullin-3 potentially functioning to recruit unidentified substrates for ubiquitination.
Figures



Similar articles
-
Ectromelia virus encodes a BTB/kelch protein, EVM150, that inhibits NF-κB signaling.J Virol. 2014 May;88(9):4853-65. doi: 10.1128/JVI.02923-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522926 Free PMC article.
-
BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase.Mol Cell Biol. 2005 Jan;25(1):162-71. doi: 10.1128/MCB.25.1.162-171.2005. Mol Cell Biol. 2005. PMID: 15601839 Free PMC article.
-
Ectromelia virus encodes a novel family of F-box proteins that interact with the SCF complex.J Virol. 2008 Oct;82(20):9917-27. doi: 10.1128/JVI.00953-08. Epub 2008 Aug 6. J Virol. 2008. PMID: 18684824 Free PMC article.
-
Interaction of orthopoxviruses with the cellular ubiquitin-ligase system.Virus Genes. 2010 Dec;41(3):309-18. doi: 10.1007/s11262-010-0519-y. Epub 2010 Aug 12. Virus Genes. 2010. PMID: 20703935 Review.
-
Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family.EMBO J. 2004 Apr 21;23(8):1681-7. doi: 10.1038/sj.emboj.7600186. Epub 2004 Apr 8. EMBO J. 2004. PMID: 15071497 Free PMC article. Review.
Cited by
-
Rotavirus NSP1 Associates with Components of the Cullin RING Ligase Family of E3 Ubiquitin Ligases.J Virol. 2016 Jun 10;90(13):6036-48. doi: 10.1128/JVI.00704-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099313 Free PMC article.
-
Inhibition of the ubiquitin-proteasome system prevents vaccinia virus DNA replication and expression of intermediate and late genes.J Virol. 2009 Mar;83(6):2469-79. doi: 10.1128/JVI.01986-08. Epub 2009 Jan 7. J Virol. 2009. PMID: 19129442 Free PMC article.
-
Progress on Poxvirus E3 Ubiquitin Ligases and Adaptor Proteins.Front Immunol. 2021 Dec 9;12:740223. doi: 10.3389/fimmu.2021.740223. eCollection 2021. Front Immunol. 2021. PMID: 34956175 Free PMC article. Review.
-
Orthopoxviruses require a functional ubiquitin-proteasome system for productive replication.J Virol. 2009 Mar;83(5):2099-108. doi: 10.1128/JVI.01753-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109393 Free PMC article.
-
The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis.J Virol. 2009 Jul;83(14):7085-98. doi: 10.1128/JVI.00437-09. Epub 2009 May 13. J Virol. 2009. PMID: 19439472 Free PMC article.
References
-
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10(1):17–24. - PubMed
-
- Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, Mayer S, Takahashi S, Licht JD, Prive GG. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell. 2003;12(6):1551–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

