Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival
- PMID: 18219273
- PMCID: PMC2241656
- DOI: 10.1038/sj.emboj.7601984
Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival
Abstract
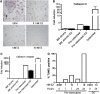
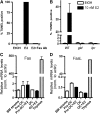
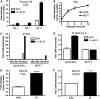
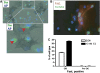
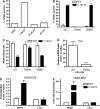
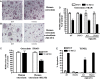
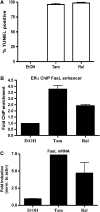
Estrogen deficiency in menopause is a major cause of osteoporosis in women. Estrogen acts to maintain the appropriate ratio between bone-forming osteoblasts and bone-resorbing osteoclasts in part through the induction of osteoclast apoptosis. Recent studies have suggested a role for Fas ligand (FasL) in estrogen-induced osteoclast apoptosis by an autocrine mechanism involving osteoclasts alone. In contrast, we describe a paracrine mechanism in which estrogen affects osteoclast survival through the upregulation of FasL in osteoblasts (and not osteoclasts) leading to the apoptosis of pre-osteoclasts. We have characterized a cell-type-specific hormone-inducible enhancer located 86 kb downstream of the FasL gene as the target of estrogen receptor-alpha induction of FasL expression in osteoblasts. In addition, tamoxifen and raloxifene, two selective estrogen receptor modulators that have protective effects in bone, induce apoptosis in pre-osteoclasts by the same osteoblast-dependent mechanism. These results demonstrate that estrogen protects bone by inducing a paracrine signal originating in osteoblasts leading to the death of pre-osteoclasts and offer an important new target for the prevention and treatment of osteoporosis.
Figures

Similar articles
-
Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass.Cell Death Differ. 2015 Oct;22(10):1654-64. doi: 10.1038/cdd.2015.14. Epub 2015 Mar 6. Cell Death Differ. 2015. PMID: 25744024 Free PMC article.
-
ERα signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis.J Bone Miner Res. 2013 Feb;28(2):283-90. doi: 10.1002/jbmr.1747. J Bone Miner Res. 2013. PMID: 22927007 Free PMC article.
-
Fas receptor is required for estrogen deficiency-induced bone loss in mice.Lab Invest. 2010 Mar;90(3):402-13. doi: 10.1038/labinvest.2009.144. Epub 2010 Jan 18. Lab Invest. 2010. PMID: 20084056 Free PMC article.
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Osteoblast-Osteoclast Communication and Bone Homeostasis.Cells. 2020 Sep 10;9(9):2073. doi: 10.3390/cells9092073. Cells. 2020. PMID: 32927921 Free PMC article. Review.
Cited by
-
A Bone Anabolic Effect of RANKL in a Murine Model of Osteoporosis Mediated Through FoxP3+ CD8 T Cells.J Bone Miner Res. 2015 Aug;30(8):1508-22. doi: 10.1002/jbmr.2472. Epub 2015 May 21. J Bone Miner Res. 2015. PMID: 25656537 Free PMC article.
-
Tissue-Specific Effects of Loss of Estrogen during Menopause and Aging.Front Endocrinol (Lausanne). 2012 Feb 8;3:19. doi: 10.3389/fendo.2012.00019. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22654856 Free PMC article.
-
Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss.Molecules. 2021 Dec 28;27(1):169. doi: 10.3390/molecules27010169. Molecules. 2021. PMID: 35011398 Free PMC article.
-
Nitric oxide and cyclic GMP functions in bone.Nitric Oxide. 2018 Jun 1;76:62-70. doi: 10.1016/j.niox.2018.03.007. Epub 2018 Mar 14. Nitric Oxide. 2018. PMID: 29550520 Free PMC article. Review.
-
Recent Advances in Osteoclast Biological Behavior.Front Cell Dev Biol. 2021 Dec 8;9:788680. doi: 10.3389/fcell.2021.788680. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957116 Free PMC article. Review.
References
-
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337–342 - PubMed
-
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43 - PubMed
-
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M (2006) Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297 - PubMed
-
- Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208: 207–227 - PubMed
-
- Cohen PL, Eisenberg RA (1991) Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 9: 243–269 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

