Chaperones in control of protein disaggregation
- PMID: 18216875
- PMCID: PMC2234349
- DOI: 10.1038/sj.emboj.7601970
Chaperones in control of protein disaggregation
Abstract
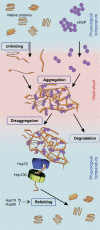
The chaperone protein network controls both initial protein folding and subsequent maintenance of proteins in the cell. Although the native structure of a protein is principally encoded in its amino-acid sequence, the process of folding in vivo very often requires the assistance of molecular chaperones. Chaperones also play a role in a post-translational quality control system and thus are required to maintain the proper conformation of proteins under changing environmental conditions. Many factors leading to unfolding and misfolding of proteins eventually result in protein aggregation. Stress imposed by high temperature was one of the first aggregation-inducing factors studied and remains one of the main models in this field. With massive protein aggregation occurring in response to heat exposure, the cell needs chaperones to control and counteract the aggregation process. Elimination of aggregates can be achieved by solubilization of aggregates and either refolding of the liberated polypeptides or their proteolysis. Here, we focus on the molecular mechanisms by which heat-shock protein 70 (Hsp70), Hsp100 and small Hsp chaperones liberate and refold polypeptides trapped in protein aggregates.
Figures
Similar articles
-
Hsp70 chaperone machine remodels protein aggregates at the initial step of Hsp70-Hsp100-dependent disaggregation.J Biol Chem. 2006 Mar 17;281(11):7022-9. doi: 10.1074/jbc.M507893200. Epub 2006 Jan 16. J Biol Chem. 2006. PMID: 16415353
-
Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network.Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):13732-7. doi: 10.1073/pnas.96.24.13732. Proc Natl Acad Sci U S A. 1999. PMID: 10570141 Free PMC article.
-
Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses.J Biol Chem. 2001 Oct 26;276(43):39586-91. doi: 10.1074/jbc.M103081200. Epub 2001 Aug 21. J Biol Chem. 2001. PMID: 11517217
-
Chaperone-assisted protein folding in the cell cytoplasm.Curr Protein Pept Sci. 2001 Sep;2(3):227-44. doi: 10.2174/1389203013381134. Curr Protein Pept Sci. 2001. PMID: 12369934 Review.
-
Cellular Handling of Protein Aggregates by Disaggregation Machines.Mol Cell. 2018 Jan 18;69(2):214-226. doi: 10.1016/j.molcel.2018.01.004. Mol Cell. 2018. PMID: 29351843 Review.
Cited by
-
CtsR regulation in mcsAB-deficient Gram-positive bacteria.J Bacteriol. 2012 Mar;194(6):1361-8. doi: 10.1128/JB.06746-11. Epub 2012 Jan 13. J Bacteriol. 2012. PMID: 22247503 Free PMC article.
-
The therapeutic efficacy of different configuration nano-polydopamine drug carrier systems with photothermal synergy against head and neck squamous cell carcinoma.Regen Biomater. 2024 Jun 20;11:rbae073. doi: 10.1093/rb/rbae073. eCollection 2024. Regen Biomater. 2024. PMID: 39027362 Free PMC article.
-
Screening strategies to identify HSP70 modulators to treat Alzheimer's disease.Drug Des Devel Ther. 2015 Jan 7;9:321-31. doi: 10.2147/DDDT.S72165. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25609918 Free PMC article. Review.
-
HSP90 Contributes to chs3-2D-Mediated Autoimmunity.Front Plant Sci. 2022 Jun 3;13:888449. doi: 10.3389/fpls.2022.888449. eCollection 2022. Front Plant Sci. 2022. PMID: 35720559 Free PMC article.
-
Hyperthermia as an immunotherapy strategy for cancer.Curr Opin Investig Drugs. 2009 Jun;10(6):550-8. Curr Opin Investig Drugs. 2009. PMID: 19513944 Free PMC article. Review.
References
-
- Albanèse V, Yen-Wen Yam A, Baughman J, Parnot C, Frydman J (2006) Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124: 75–88 - PubMed
-
- Ammelburg M, Frickey T, Lupas AN (2006) Classification of AAA+ proteins. J Struct Biol 156: 2–11 - PubMed
-
- Barnett ME, Nagy M, Kedzierska S, Zolkiewski M (2005) The amino-terminal domain of ClpB supports binding to strongly aggregated proteins. J Biol Chem 280: 34940–34945 - PubMed
-
- Basha E, Lee GJ, Breci LA, Hausrath AC, Buan NR, Giese KC, Vierling E (2004) The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J Biol Chem 279: 7566–7575 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

