Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence
- PMID: 18207746
- PMCID: PMC2997702
- DOI: 10.1016/j.cub.2007.12.057
Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence
Abstract
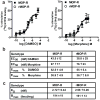
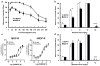
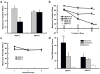
Opioid drugs, such as morphine, are among the most effective analgesics available. However, their utility for the treatment of chronic pain is limited by side effects including tolerance and dependence. Morphine acts primarily through the mu-opioid receptor (MOP-R) , which is also a target of endogenous opioids. However, unlike endogenous ligands, morphine fails to promote substantial receptor endocytosis both in vitro, and in vivo. Receptor endocytosis serves at least two important functions in signal transduction. First, desensitization and endocytosis act as an "off" switch by uncoupling receptors from G protein. Second, endocytosis functions as an "on" switch, resensitizing receptors by recycling them to the plasma membrane. Thus, both the off and on function of the MOP-R are altered in response to morphine compared to endogenous ligands. To examine whether the low degree of endocytosis induced by morphine contributes to tolerance and dependence, we generated a knockin mouse that expresses a mutant MOP-R that undergoes morphine-induced endocytosis. Morphine remains an excellent antinociceptive agent in these mice. Importantly, these mice display substantially reduced antinociceptive tolerance and physical dependence. These data suggest that opioid drugs with a pharmacological profile similar to morphine but the ability to promote endocytosis could provide analgesia while having a reduced liability for promoting tolerance and dependence.
Figures

Similar articles
-
The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence.Curr Opin Neurobiol. 2007 Oct;17(5):556-64. doi: 10.1016/j.conb.2007.10.004. Epub 2007 Dec 18. Curr Opin Neurobiol. 2007. PMID: 18068348 Review.
-
An opiate cocktail that reduces morphine tolerance and dependence.Curr Biol. 2005 Jun 7;15(11):1028-33. doi: 10.1016/j.cub.2005.04.052. Curr Biol. 2005. PMID: 15936273
-
Prohormone convertase 2 (PC2) null mice have increased mu opioid receptor levels accompanied by altered morphine-induced antinociception, tolerance and dependence.Neuroscience. 2016 Aug 4;329:318-25. doi: 10.1016/j.neuroscience.2016.05.021. Epub 2016 May 18. Neuroscience. 2016. PMID: 27208618 Free PMC article.
-
micro-Opioid receptor endocytosis prevents adaptations in ventral tegmental area GABA transmission induced during naloxone-precipitated morphine withdrawal.J Neurosci. 2010 Mar 3;30(9):3276-86. doi: 10.1523/JNEUROSCI.4634-09.2010. J Neurosci. 2010. PMID: 20203187 Free PMC article.
-
Opioid regulation of mu receptor internalisation: relevance to the development of tolerance and dependence.CNS Neurol Disord Drug Targets. 2010 Nov;9(5):616-26. doi: 10.2174/187152710793361522. CNS Neurol Disord Drug Targets. 2010. PMID: 20632966 Review.
Cited by
-
Pharmacological and genetic manipulations at the µ-opioid receptor reveal arrestin-3 engagement limits analgesic tolerance and does not exacerbate respiratory depression in mice.Neuropsychopharmacology. 2021 Dec;46(13):2241-2249. doi: 10.1038/s41386-021-01054-x. Epub 2021 Jul 13. Neuropsychopharmacology. 2021. PMID: 34257415 Free PMC article.
-
Opioid-receptor-heteromer-specific trafficking and pharmacology.Curr Opin Pharmacol. 2010 Feb;10(1):73-9. doi: 10.1016/j.coph.2009.09.007. Epub 2009 Oct 19. Curr Opin Pharmacol. 2010. PMID: 19846340 Free PMC article. Review.
-
Prolonged morphine treatment alters δ opioid receptor post-internalization trafficking.Br J Pharmacol. 2015 Jan;172(2):615-29. doi: 10.1111/bph.12761. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24819092 Free PMC article.
-
Synthesis of the Mechanisms of Opioid Tolerance: Do We Still Say NO?Cell Mol Neurobiol. 2021 Jul;41(5):927-948. doi: 10.1007/s10571-021-01065-8. Epub 2021 Mar 11. Cell Mol Neurobiol. 2021. PMID: 33704603 Review.
-
Regulation of opioid receptors by endocytic membrane traffic: mechanisms and translational implications.Drug Alcohol Depend. 2010 May 1;108(3):166-71. doi: 10.1016/j.drugalcdep.2010.02.014. Epub 2010 Mar 24. Drug Alcohol Depend. 2010. PMID: 20338697 Free PMC article. Review.
References
-
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. - PubMed
-
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. - PubMed
-
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von ZM. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. - PubMed
-
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V. C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem. 2001;276:31408–31414. - PubMed
-
- Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB. Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem. 1997;272:28869–28874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

