Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis
- PMID: 18202187
- PMCID: PMC2312353
- DOI: 10.2353/ajpath.2008.070726
Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis
Abstract
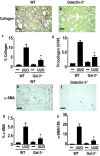
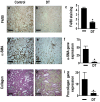
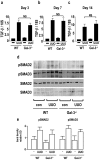
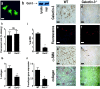
Macrophages have been proposed as a key cell type in the pathogenesis of renal fibrosis; however, the mechanism by which macrophages drive fibrosis is still unclear. We show that expression of galectin-3, a beta-galactoside-binding lectin, is up-regulated in a mouse model of progressive renal fibrosis (unilateral ureteric obstruction, UUO), and absence of galectin-3 protects against renal myofibroblast accumulation/activation and fibrosis. Furthermore, specific depletion of macrophages using CD11b-DTR mice reduces fibrosis severity after UUO demonstrating that macrophages are key cells in the pathogenesis of renal fibrosis. Disruption of the galectin-3 gene does not affect macrophage recruitment after UUO, or macrophage proinflammatory cytokine profiles in response to interferon-gamma/lipopolysaccharide. In addition, absence of galectin-3 does not affect transforming growth factor-beta expression or Smad 2/3 phosphorylation in obstructed kidneys. Adoptive transfer of wild-type but not galectin-3(-/-) macrophages did, however, restore the fibrotic phenotype in galectin-3(-/-) mice. Cross-over experiments using wild-type and galectin-3(-/-) macrophage supernatants and renal fibroblasts confirmed that secretion of galectin-3 by macrophages is critical in the activation of renal fibroblasts to a profibrotic phenotype. Therefore, we demonstrate for the first time that galectin-3 expression and secretion by macrophages is a major mechanism linking macrophages to the promotion of renal fibrosis.
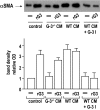
Figures



Similar articles
-
Twist1 regulates macrophage plasticity to promote renal fibrosis through galectin-3.Cell Mol Life Sci. 2022 Feb 19;79(3):137. doi: 10.1007/s00018-022-04137-0. Cell Mol Life Sci. 2022. PMID: 35182235 Free PMC article.
-
Inhibitory effects of fasudil on renal interstitial fibrosis induced by unilateral ureteral obstruction.Mol Med Rep. 2015 Dec;12(6):8010-20. doi: 10.3892/mmr.2015.4467. Epub 2015 Oct 21. Mol Med Rep. 2015. PMID: 26498136 Free PMC article.
-
Role of galectin-3 in human pulmonary fibrosis.Allergol Int. 2007 Mar;56(1):57-65. doi: 10.2332/allergolint.O-06-449. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259811
-
Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target.Int J Mol Sci. 2022 Mar 14;23(6):3124. doi: 10.3390/ijms23063124. Int J Mol Sci. 2022. PMID: 35328545 Free PMC article. Review.
-
The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis.Int J Mol Sci. 2024 Jul 25;25(15):8129. doi: 10.3390/ijms25158129. Int J Mol Sci. 2024. PMID: 39125698 Free PMC article. Review.
Cited by
-
Macrophage and epithelial cell H-ferritin expression regulates renal inflammation.Kidney Int. 2015 Jul;88(1):95-108. doi: 10.1038/ki.2015.102. Epub 2015 Apr 15. Kidney Int. 2015. PMID: 25874599 Free PMC article.
-
Downregulating galectin-3 inhibits proinflammatory cytokine production by human monocyte-derived dendritic cells via RNA interference.Cell Immunol. 2015 Mar;294(1):44-53. doi: 10.1016/j.cellimm.2015.01.017. Epub 2015 Feb 7. Cell Immunol. 2015. PMID: 25684095 Free PMC article.
-
Galectin-3-A New Player of Kidney Damage or an Innocent Bystander in Children with a Single Kidney?J Clin Med. 2021 May 8;10(9):2012. doi: 10.3390/jcm10092012. J Clin Med. 2021. PMID: 34066698 Free PMC article.
-
Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes.JACC Basic Transl Sci. 2016 Apr;1(3):143-154. doi: 10.1016/j.jacbts.2016.03.003. JACC Basic Transl Sci. 2016. PMID: 27525318 Free PMC article.
-
Macrophages: master regulators of inflammation and fibrosis.Semin Liver Dis. 2010 Aug;30(3):245-57. doi: 10.1055/s-0030-1255354. Epub 2010 Jul 21. Semin Liver Dis. 2010. PMID: 20665377 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

