Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells
- PMID: 18199645
- PMCID: PMC2258971
- DOI: 10.1128/JVI.02456-07
Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells
Abstract
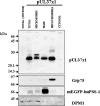
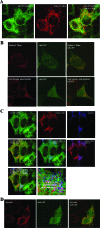
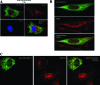
The human cytomegalovirus (HCMV) UL37 exon 1 protein (pUL37x1), also known as vMIA, is the predominant UL37 isoform during permissive infection. pUL37x1 is a potent antiapoptotic protein, which prevents cytochrome c release from mitochondria. The UL37x1 NH(2)-terminal bipartite localization signal, which remains uncleaved, targets UL37 proteins to the endoplasmic reticulum (ER) and then to mitochondria. Based upon our findings, we hypothesized that pUL37x1 traffics from the ER to mitochondria through direct contacts between the two organelles, provided by mitochondrion-associated membranes (MAMs). To facilitate its identification, we cloned and tagged the human phosphatidylserine synthase 1 (huPSS-1) cDNA, whose mouse homologue localizes almost exclusively in the MAM. Using subcellular fractionation of stable HeLa cell transfectants expressing mEGFP-huPSS-1, we found that HCMV pUL37x1 is present in purified microsomes, mitochondria, and MAM fractions. We further examined the trafficking of the full-length UL37 glycoprotein cleavage products, which divergently traffic either through the secretory apparatus or into mitochondria. Surprisingly, pUL37(NH2) and gpUL37(COOH) were both detected in the ER and MAM fraction, even though only pUL37(NH2) is preferentially imported into mitochondria but gpUL37(COOH) is not. To determine the sequences required for MAM importation, we examined pUL37x1 mutants that were partially defective for mitochondrial importation. Deletion mutants of the NH(2)-terminal UL37x1 mitochondrial localization signal were reduced in trafficking into the MAM, indicating partial overlap of MAM and mitochondrial targeting signals. Taken together, these results suggest that HCMV UL37 proteins traffic from the ER into the MAM, where they are sorted into either the secretory pathway or to mitochondrial importation.
Figures
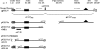



Similar articles
-
Processing of human cytomegalovirus UL37 mutant glycoproteins in the endoplasmic reticulum lumen prior to mitochondrial importation.J Virol. 2006 Jul;80(14):6771-83. doi: 10.1128/JVI.00492-06. J Virol. 2006. PMID: 16809283 Free PMC article.
-
The human cytomegalovirus protein UL37 exon 1 associates with internal lipid rafts.J Virol. 2011 Mar;85(5):2100-11. doi: 10.1128/JVI.01830-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177823 Free PMC article.
-
Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection.J Gen Virol. 2004 Feb;85(Pt 2):323-329. doi: 10.1099/vir.0.19589-0. J Gen Virol. 2004. PMID: 14769889
-
Superresolution imaging of viral protein trafficking.Med Microbiol Immunol. 2015 Jun;204(3):449-60. doi: 10.1007/s00430-015-0395-0. Epub 2015 Feb 28. Med Microbiol Immunol. 2015. PMID: 25724304 Free PMC article. Review.
-
Access of viral proteins to mitochondria via mitochondria-associated membranes.Rev Med Virol. 2009 May;19(3):147-64. doi: 10.1002/rmv.611. Rev Med Virol. 2009. PMID: 19367604 Free PMC article. Review.
Cited by
-
Mitochondria and Peroxisome Remodeling across Cytomegalovirus Infection Time Viewed through the Lens of Inter-ViSTA.Cell Rep. 2020 Jul 28;32(4):107943. doi: 10.1016/j.celrep.2020.107943. Cell Rep. 2020. PMID: 32726614 Free PMC article.
-
Superresolution Imaging Identifies That Conventional Trafficking Pathways Are Not Essential for Endoplasmic Reticulum to Outer Mitochondrial Membrane Protein Transport.Sci Rep. 2017 Feb 2;7(1):16. doi: 10.1038/s41598-017-00039-5. Sci Rep. 2017. PMID: 28154412 Free PMC article.
-
Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection.J Virol. 2020 Jan 6;94(2):e01183-19. doi: 10.1128/JVI.01183-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694945 Free PMC article.
-
Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection.Mol Cell Proteomics. 2011 Oct;10(10):M111.009936. doi: 10.1074/mcp.M111.009936. Epub 2011 Jul 8. Mol Cell Proteomics. 2011. PMID: 21742798 Free PMC article.
-
Isolation of Endoplasmic Reticulum, Mitochondria, and Mitochondria-Associated Membrane and Detergent Resistant Membrane Fractions from Transfected Cells and from Human Cytomegalovirus-Infected Primary Fibroblasts.Curr Protoc Cell Biol. 2015 Sep 1;68:3.27.1-3.27.33. doi: 10.1002/0471143030.cb0327s68. Curr Protoc Cell Biol. 2015. PMID: 26331984 Free PMC article.
References
-
- Adair, R., G. W. Liebisch, and A. M. Colberg-Poley. 2003. Complex alternative processing of human cytomegalovirus UL37 pre-mRNA. J. Gen. Virol. 843353-3358. - PubMed
-
- Anandatheerthavarada, H. K., G. Biswas, J. Mullick, N. B. Sepuri, L. Otvos, D. Pain, and N. G. Avadhani. 1999. Dual targeting of cytochrome P4502B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at ser128. EMBO J. 185494-5504. - PMC - PubMed
-
- Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 1017988-7993. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

