Crosstalk between the alpha2beta1 integrin and c-met/HGF-R regulates innate immunity
- PMID: 18198349
- PMCID: PMC2275022
- DOI: 10.1182/blood-2007-08-107664
Crosstalk between the alpha2beta1 integrin and c-met/HGF-R regulates innate immunity
Abstract
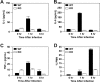
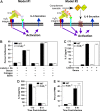
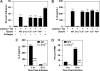
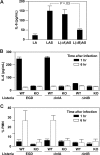
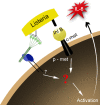
Data from several investigators suggest that the alpha2beta1 integrin, a receptor for collagens, laminins, decorin, E-cadherin, matrix metalloproteinase-1, endorepellin, and several viruses, is required for innate immunity and regulation of autoimmune/allergic disorders. We demonstrated that the innate immune response to Listeria monocytogenes required alpha2beta1 integrin expression by peritoneal mast cells (PMCs). Ligation of the alpha2beta1 integrin by C1q contained in immune complexes comprised of Listeria and antibody was required for PMC activation in vitro and in vivo. However, ligation of the alpha2beta1 integrin alone was insufficient to activate cytokine secretion, suggesting that one or more additional signals emanating from a coreceptor were required for PMC activation. Here, we demonstrate that C1q, but neither other complement proteins nor FcRgamma, is required for early innate immune response to Listeria. The binding of Listeria's Internalin B (InlB) to hepatocyte growth factor receptor (HGF-R)/c-met provides the costimulatory function required for PMC activation. Either HGF or Listeria InlB bound to c-met and either C1q or type I collagen bound to alpha2beta1 integrin stimulates PMC activation. These findings suggest that crosstalk between c-met and the alpha2beta1 integrin may contribute to mast-cell activation in autoimmune and inflammatory disorders.
Figures

Similar articles
-
Novel collectin/C1q receptor mediates mast cell activation and innate immunity.Blood. 2006 Jan 1;107(1):143-50. doi: 10.1182/blood-2005-06-2218. Epub 2005 Sep 15. Blood. 2006. PMID: 16166590 Free PMC article.
-
The alpha2beta1 integrin: a novel collectin/C1q receptor.Immunobiology. 2007;212(4-5):343-53. doi: 10.1016/j.imbio.2006.11.013. Epub 2007 Jan 2. Immunobiology. 2007. PMID: 17544819 Review.
-
Selective, α2β1 integrin-dependent secretion of il-6 by connective tissue mast cells.J Innate Immun. 2011;3(5):459-70. doi: 10.1159/000324832. Epub 2011 Apr 19. J Innate Immun. 2011. PMID: 21502744 Free PMC article.
-
Mast cell-mediated inflammatory responses require the alpha 2 beta 1 integrin.Blood. 2004 Mar 15;103(6):2214-20. doi: 10.1182/blood-2003-08-2978. Epub 2003 Nov 26. Blood. 2004. PMID: 14645004
-
Structural basis of MET receptor dimerization by the bacterial invasion protein InlB and the HGF/SF splice variant NK1.Biochim Biophys Acta. 2013 Oct;1834(10):2195-204. doi: 10.1016/j.bbapap.2012.10.012. Epub 2012 Oct 31. Biochim Biophys Acta. 2013. PMID: 23123275 Review.
Cited by
-
Peroxisome proliferator-activated receptor γ (PPARγ) induces the gene expression of integrin αVβ5 to promote macrophage M2 polarization.J Biol Chem. 2018 Oct 26;293(43):16572-16582. doi: 10.1074/jbc.RA118.003161. Epub 2018 Sep 4. J Biol Chem. 2018. PMID: 30181212 Free PMC article.
-
Similarities in features of autism and asthma and a possible link to acetaminophen use.Med Hypotheses. 2010 Jan;74(1):7-11. doi: 10.1016/j.mehy.2009.08.033. Epub 2009 Sep 11. Med Hypotheses. 2010. PMID: 19748189 Free PMC article.
-
Association of integrin beta1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer.Cancer Cell Int. 2013 Feb 13;13(1):15. doi: 10.1186/1475-2867-13-15. Cancer Cell Int. 2013. PMID: 23402326 Free PMC article.
-
Regulation and functions of integrin α2 in cell adhesion and disease.Genes Dis. 2018 Dec 31;6(1):16-24. doi: 10.1016/j.gendis.2018.12.003. eCollection 2019 Mar. Genes Dis. 2018. PMID: 30906828 Free PMC article. Review.
-
The Pleiotropic MET Receptor Network: Circuit Development and the Neural-Medical Interface of Autism.Biol Psychiatry. 2017 Mar 1;81(5):424-433. doi: 10.1016/j.biopsych.2016.08.035. Epub 2016 Sep 15. Biol Psychiatry. 2017. PMID: 27837921 Free PMC article. Review.
References
-
- Edelson BT, Li Z, Pappan LK, Zutter MM. Mast cell-mediated inflammatory responses require the α2β1 integrin. Blood. 2004;103:2214–2220. - PubMed
-
- Zutter MM. Function of the alpha2 beta1 integrin. In: Gullberg D, editor. I Domains in Integrins. Georgetown, TX: Landis Bioscience; 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

