Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus)
- PMID: 18198149
- PMCID: PMC2596896
- DOI: 10.1098/rspb.2007.1260
Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus)
Abstract
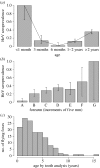
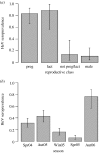
Hendra virus (HeV) is a lethal paramyxovirus which emerged in humans in 1994. Poor understanding of HeV dynamics in Pteropus spp. (flying fox or fruit bat) reservoir hosts has limited our ability to determine factors driving its emergence. We initiated a longitudinal field study of HeV in little red flying foxes (LRFF; Pteropus scapulatus) and examined individual and population risk factors for infection, to determine probable modes of intraspecific transmission. We also investigated whether seasonal changes in host behaviour, physiology and demography affect host-pathogen dynamics. Data showed that pregnant and lactating females had significantly higher risk of infection, which may explain previously observed temporal associations between HeV outbreaks and flying fox birthing periods. Age-specific seroprevalence curves generated from field data imply that HeV is transmitted horizontally via faeces, urine or saliva. Rapidly declining seroprevalence between two field seasons suggests that immunity wanes faster in LRFF than in other flying fox species, and highlights the potentially critical role of this species in interspecific viral persistence. The highest seroprevalence was observed when animals showed evidence of nutritional stress, suggesting that environmental processes that alter flying fox food sources, such as habitat loss and climate change, may increase HeV infection and transmission. These insights into the ecology of HeV in flying fox populations suggest causal links between anthropogenic environmental change and HeV emergence.
Figures

Similar articles
-
Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.).Proc Biol Sci. 2011 Dec 22;278(1725):3703-12. doi: 10.1098/rspb.2011.0522. Epub 2011 May 11. Proc Biol Sci. 2011. PMID: 21561971 Free PMC article.
-
Routes of Hendra Virus Excretion in Naturally-Infected Flying-Foxes: Implications for Viral Transmission and Spillover Risk.PLoS One. 2015 Oct 15;10(10):e0140670. doi: 10.1371/journal.pone.0140670. eCollection 2015. PLoS One. 2015. PMID: 26469523 Free PMC article.
-
Hendra Virus Infection Dynamics in the Grey-Headed Flying Fox (Pteropus poliocephalus) at the Southern-Most Extent of Its Range: Further Evidence This Species Does Not Readily Transmit the Virus to Horses.PLoS One. 2016 Jun 15;11(6):e0155252. doi: 10.1371/journal.pone.0155252. eCollection 2016. PLoS One. 2016. PMID: 27304985 Free PMC article.
-
Hendra virus ecology and transmission.Curr Opin Virol. 2016 Feb;16:120-125. doi: 10.1016/j.coviro.2016.02.004. Epub 2016 Mar 12. Curr Opin Virol. 2016. PMID: 26978066 Review.
-
Henipaviruses: an updated review focusing on the pteropid reservoir and features of transmission.Zoonoses Public Health. 2013 Feb;60(1):69-83. doi: 10.1111/j.1863-2378.2012.01501.x. Epub 2012 Jun 18. Zoonoses Public Health. 2013. PMID: 22709528 Review.
Cited by
-
Bats generate lower affinity but higher diversity antibody responses than those of mice, but pathogen-binding capacity increases if protein is restricted in their diet.PLoS Biol. 2024 Sep 24;22(9):e3002800. doi: 10.1371/journal.pbio.3002800. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39316608 Free PMC article.
-
A tale of endurance: bats, viruses and immune dynamics.Future Microbiol. 2024 Jun 12;19(9):841-856. doi: 10.2217/fmb-2023-0233. Epub 2024 Apr 22. Future Microbiol. 2024. PMID: 38648093 Review.
-
Advances in understanding bat infection dynamics across biological scales.Proc Biol Sci. 2024 Mar 13;291(2018):20232823. doi: 10.1098/rspb.2023.2823. Epub 2024 Mar 6. Proc Biol Sci. 2024. PMID: 38444339 Free PMC article. Review.
-
Coordinated inflammatory responses dictate Marburg virus control by reservoir bats.Nat Commun. 2024 Feb 28;15(1):1826. doi: 10.1038/s41467-024-46226-7. Nat Commun. 2024. PMID: 38418477 Free PMC article.
-
Selective replication and vertical transmission of Ebola virus in experimentally infected Angolan free-tailed bats.Nat Commun. 2024 Jan 31;15(1):925. doi: 10.1038/s41467-024-45231-0. Nat Commun. 2024. PMID: 38297087 Free PMC article.
References
-
- Alexander J, Stimson W.H. Sex-hormones and the course of parasitic infection. Parasitol. Today. 1988;4:189–193. doi:10.1016/0169-4758(88)90077-4 - DOI
-
- Bastian S.T, Jr, Tanaka K, Anunciado R.V.P, Natural N.G, Sumalde A.C, Namikawa T. Evolutionary relationships of flying foxes (Genus Pteropus) in The Philippines inferred from DNA sequences of cytochrome b gene. Biochem. Genet. 2002;40:101–116. doi:10.1023/A:1015161305843 - DOI - PubMed
-
- Birt, P. 2004 Mutualistic interactions between the nectar-feeding little red flying fox Pteropus scapulatus (Chiroptera: Pteropodidae) and flowering eucalypts (Myrtaceae): habitat utilisation and pollination. PhD thesis, University of Queensland, Brisbane, Australia.
-
- Bossart K.N, McEachern J.A, Hickey A.C, Choudhry V, Dimitrov D.S, Eaton B.T, Wang L.F. Neutralization assays for differential henipavirus serology using Bio-Plex protein array systems. J. Virol. Methods. 2007;142:29–40. doi:10.1016/j.jviromet.2007.01.003 - DOI - PubMed
-
- Cattadori I.M, Boag B, Bjornstad O.N, Cornell S.J, Hudson P.J. Peak shift and epidemiology in a seasonal host-nematode system. Proc. R. Soc. B. 2005;272:1163–1169. doi:10.1098/rspb.2004.3050 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

