Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses
- PMID: 18195069
- PMCID: PMC2234357
- DOI: 10.1084/jem.20062027
Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses
Abstract
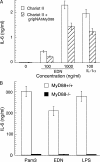
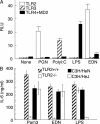
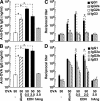
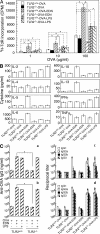
Eosinophil-derived neurotoxin (EDN) is an eosinophil granule-derived secretory protein with ribonuclease and antiviral activity. We have previously shown that EDN can induce the migration and maturation of dendritic cells (DCs). Here, we report that EDN can activate myeloid DCs by triggering the Toll-like receptor (TLR)2-myeloid differentiation factor 88 signaling pathway, thus establishing EDN as an endogenous ligand of TLR2. EDN activates TLR2 independently of TLR1 or TLR6. When mice were immunized with ovalbumin (OVA) together with EDN or with EDN-treated OVA-loaded DCs, EDN enhanced OVA-specific T helper (Th)2-biased immune responses as indicated by predominant production of OVA-specific interleukin (IL)-5, IL-6, IL-10, and IL-13, as well as higher levels of immunoglobulin (Ig)G1 than IgG2a. Based on its ability to serve as a chemoattractant and activator of DCs, as well as the capacity to enhance antigen-specific immune responses, we consider EDN to have the properties of an endogenous alarmin that alerts the adaptive immune system for preferential enhancement of antigen-specific Th2 immune responses.
Figures




Comment in
-
Alarming dendritic cells for Th2 induction.J Exp Med. 2008 Jan 21;205(1):13-7. doi: 10.1084/jem.20072665. Epub 2008 Jan 14. J Exp Med. 2008. PMID: 18195077 Free PMC article.
Similar articles
-
Roles of lipoxin A4 receptor activation and anti-interleukin-1β antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-κB pathway in airway inflammation induced by ovalbumin.Mol Med Rep. 2015 Jul;12(1):895-904. doi: 10.3892/mmr.2015.3443. Epub 2015 Mar 5. Mol Med Rep. 2015. PMID: 25760938 Free PMC article.
-
Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation.J Immunol. 2004 Nov 15;173(10):6134-42. doi: 10.4049/jimmunol.173.10.6134. J Immunol. 2004. PMID: 15528350 Free PMC article.
-
Desert dust induces TLR signaling to trigger Th2-dominant lung allergic inflammation via a MyD88-dependent signaling pathway.Toxicol Appl Pharmacol. 2016 Apr 1;296:61-72. doi: 10.1016/j.taap.2016.02.011. Epub 2016 Feb 13. Toxicol Appl Pharmacol. 2016. PMID: 26882889
-
Eosinophil-derived neurotoxin / RNase 2: connecting the past, the present and the future.Curr Pharm Biotechnol. 2008 Jun;9(3):135-40. doi: 10.2174/138920108784567236. Curr Pharm Biotechnol. 2008. PMID: 18673278 Free PMC article. Review.
-
Eosinophil-Derived Neurotoxin (EDN/RNase 2) and the Mouse Eosinophil-Associated RNases (mEars): Expanding Roles in Promoting Host Defense.Int J Mol Sci. 2015 Jul 8;16(7):15442-55. doi: 10.3390/ijms160715442. Int J Mol Sci. 2015. PMID: 26184157 Free PMC article. Review.
Cited by
-
Participation of MyD88 and interleukin-33 as innate drivers of Th2 immunity to Trichinella spiralis.Infect Immun. 2013 Apr;81(4):1354-63. doi: 10.1128/IAI.01307-12. Epub 2013 Feb 12. Infect Immun. 2013. PMID: 23403558 Free PMC article.
-
High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses.J Exp Med. 2012 Jan 16;209(1):157-71. doi: 10.1084/jem.20101354. Epub 2011 Dec 19. J Exp Med. 2012. PMID: 22184635 Free PMC article.
-
Eosinophil-associated ribonuclease 11 is a macrophage chemoattractant.J Biol Chem. 2015 Apr 3;290(14):8863-75. doi: 10.1074/jbc.M114.626648. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713137 Free PMC article.
-
DAMPening inflammation by modulating TLR signalling.Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. Epub 2010 Jul 13. Mediators Inflamm. 2010. PMID: 20706656 Free PMC article. Review.
-
Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment.Front Immunol. 2013 Dec 10;4:436. doi: 10.3389/fimmu.2013.00436. Front Immunol. 2013. PMID: 24339825 Free PMC article. Review.
References
-
- Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. - PubMed
-
- Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710–720. - PubMed
-
- Yang, D., A. Biragyn, D.M. Hoover, J. Lubkowski, and J.J. Oppenheim. 2004. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22:181–315. - PubMed
-
- Yang, D., and J.J. Oppenheim. 2004. Antimicrobial proteins act as “alarmins” in joint immune defense. Arthritis Rheum. 50:3401–3403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

