Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development
- PMID: 18194655
- PMCID: PMC2709787
- DOI: 10.1016/j.devcel.2007.11.002
Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development
Abstract
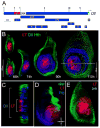
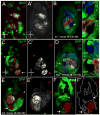
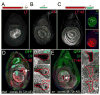
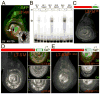
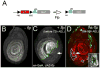
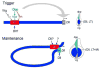
The development of the Drosophila leg requires both Decapentaplegic (Dpp) and Wingless (Wg), two signals that establish the proximo-distal (PD) axis by activating target genes such as Distalless (Dll). Dll expression in the leg depends on a Dpp- and Wg-dependent phase and a maintenance phase that is independent of these signals. Here, we show that accurate Dll expression in the leg results from the synergistic interaction between two cis-regulatory elements. The Leg Trigger (LT) element directly integrates Wg and Dpp inputs and is only active in cells receiving high levels of both signals. The Maintenance (M) element is able to maintain Wg- and Dpp-independent expression, but only when in cis to LT. M, which includes the native Dll promoter, functions as an autoregulatory element by directly binding Dll. The "trigger-maintenance" model describes a mechanism by which secreted morphogens act combinatorially to induce the stable expression of target genes.
Figures

Similar articles
-
Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg.Development. 2008 Feb;135(4):627-36. doi: 10.1242/dev.014670. Epub 2008 Jan 9. Development. 2008. PMID: 18184724 Free PMC article.
-
Establishment of medial fates along the proximodistal axis of the Drosophila leg through direct activation of dachshund by Distalless.Dev Cell. 2011 Apr 19;20(4):455-68. doi: 10.1016/j.devcel.2011.03.017. Dev Cell. 2011. PMID: 21497759 Free PMC article.
-
A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling.Development. 1998 Jan;125(1):115-24. doi: 10.1242/dev.125.1.115. Development. 1998. PMID: 9389669
-
A dynamic network of morphogens and transcription factors patterns the fly leg.Curr Top Dev Biol. 2012;98:173-98. doi: 10.1016/B978-0-12-386499-4.00007-0. Curr Top Dev Biol. 2012. PMID: 22305163 Free PMC article. Review.
-
[Morphogens and endocytosis].Med Sci (Paris). 2003 Mar;19(3):351-7. doi: 10.1051/medsci/2003193351. Med Sci (Paris). 2003. PMID: 12836418 Review. French.
Cited by
-
Role of the Forkhead Transcription Factors Fd4 and Fd5 During Drosophila Leg Development.Front Cell Dev Biol. 2021 Aug 2;9:723927. doi: 10.3389/fcell.2021.723927. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34409041 Free PMC article.
-
Scaling the Drosophila Wing: TOR-Dependent Target Gene Access by the Hippo Pathway Transducer Yorkie.PLoS Biol. 2015 Oct 16;13(10):e1002274. doi: 10.1371/journal.pbio.1002274. eCollection 2015 Oct. PLoS Biol. 2015. PMID: 26474042 Free PMC article.
-
Interrogating the function of metazoan histones using engineered gene clusters.Dev Cell. 2015 Feb 9;32(3):373-86. doi: 10.1016/j.devcel.2014.12.025. Dev Cell. 2015. PMID: 25669886 Free PMC article.
-
Robust Wnt signaling is maintained by a Wg protein gradient and Fz2 receptor activity in the developing Drosophila wing.Development. 2019 Aug 9;146(15):dev174789. doi: 10.1242/dev.174789. Development. 2019. PMID: 31399474 Free PMC article.
-
Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg.Development. 2008 Feb;135(4):627-36. doi: 10.1242/dev.014670. Epub 2008 Jan 9. Development. 2008. PMID: 18184724 Free PMC article.
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
-
- Arnosti DN. Analysis and function of transcriptional regulatory elements: insights from Drosophila. Annu Rev Entomol. 2003;48:579–602. - PubMed
-
- Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. - PubMed
-
- Breiling A, Sessa L, Orlando V. Biology of polycomb and trithorax group proteins. Int Rev Cytol. 2007;258:83–136. - PubMed
-
- Brock HW, Fisher CL. Maintenance of gene expression patterns. Dev Dyn. 2005;232:633–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

