Knockout of beta(1)- and beta(2)-adrenoceptors attenuates pressure overload-induced cardiac hypertrophy and fibrosis
- PMID: 18193078
- PMCID: PMC2259198
- DOI: 10.1038/sj.bjp.0707622
Knockout of beta(1)- and beta(2)-adrenoceptors attenuates pressure overload-induced cardiac hypertrophy and fibrosis
Abstract
Background and purpose: The role of beta-adrenoceptors in heart disease remains controversial. Although beta-blockers ameliorate the progression of heart disease, the mechanism remains undefined. We investigated the effect of beta-adrenoceptors on cardiac hypertrophic growth using beta(1)- and beta(2)-adrenoreceptor knockout and wild-type (WT) mice.
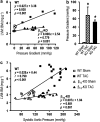
Experimental approach: Mice were subjected to aortic banding or sham surgery, and their cardiac function was determined by echocardiography and micromanometry.
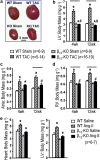
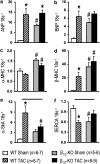
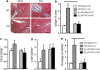
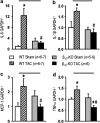
Key results: At 4 and 12 weeks after aortic banding, the left ventricle:body mass ratio was increased by 80-87% in wild-type mice, but only by 15% in knockouts, relative to sham-operated groups. Despite the blunted hypertrophic growth, ventricular function in knockouts was maintained. WT mice responded to pressure overload with up-regulation of gene expression of inflammatory cytokines and fibrogenic growth factors, and with severe cardiac fibrosis. All these effects were absent in the knockout animals.
Conclusion and implications: Our findings of a markedly attenuated cardiac hypertrophy and fibrosis following pressure overload in this knockout model emphasize that beta-adrenoceptor signalling plays a central role in cardiac hypertrophy and maladaptation following pressure overload.
Figures
Similar articles
-
Pressure overload causes cardiac hypertrophy in beta1-adrenergic and beta2-adrenergic receptor double knockout mice.J Hypertens. 2006 Mar;24(3):563-71. doi: 10.1097/01.hjh.0000203843.41937.2a. J Hypertens. 2006. PMID: 16467660
-
Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state.Circulation. 2007 Mar 20;115(11):1398-407. doi: 10.1161/CIRCULATIONAHA.106.643585. Epub 2007 Mar 12. Circulation. 2007. PMID: 17353445
-
Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload.J Am Coll Cardiol. 2006 Feb 21;47(4):817-26. doi: 10.1016/j.jacc.2005.09.051. Epub 2006 Jan 26. J Am Coll Cardiol. 2006. PMID: 16487851
-
Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure.Clin Exp Pharmacol Physiol. 2009 Nov;36(11):1054-61. doi: 10.1111/j.1440-1681.2009.05243.x. Epub 2009 Jun 29. Clin Exp Pharmacol Physiol. 2009. PMID: 19566828
-
Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart.J Steroid Biochem Mol Biol. 2013 Jul;136:150-5. doi: 10.1016/j.jsbmb.2012.09.007. Epub 2012 Sep 16. J Steroid Biochem Mol Biol. 2013. PMID: 22989481 Review.
Cited by
-
Proliferation in cardiac fibroblasts induced by β1-adrenoceptor autoantibody and the underlying mechanisms.Sci Rep. 2016 Aug 31;6:32430. doi: 10.1038/srep32430. Sci Rep. 2016. PMID: 27577254 Free PMC article.
-
Sympatho-adrenergic mechanisms in heart failure: new insights into pathophysiology.Med Rev (2021). 2021 Oct 21;1(1):47-77. doi: 10.1515/mr-2021-0007. eCollection 2021 Oct. Med Rev (2021). 2021. PMID: 37724075 Free PMC article.
-
A metabolite of Danshen formulae attenuates cardiac fibrosis induced by isoprenaline, via a NOX2/ROS/p38 pathway.Br J Pharmacol. 2015 Dec;172(23):5573-85. doi: 10.1111/bph.13133. Epub 2015 May 5. Br J Pharmacol. 2015. PMID: 25766073 Free PMC article.
-
Early activation of the cardiac CX3CL1/CX3CR1 axis delays β-adrenergic-induced heart failure.Sci Rep. 2021 Sep 9;11(1):17982. doi: 10.1038/s41598-021-97493-z. Sci Rep. 2021. PMID: 34504250 Free PMC article.
-
Cardiac pressure overload hypertrophy is differentially regulated by β-adrenergic receptor subtypes.Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1461-70. doi: 10.1152/ajpheart.00453.2010. Epub 2011 Jun 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21705675 Free PMC article.
References
-
- Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ Res. 2001;89:997–1004. - PubMed
-
- Bernstein D. Cardiovascular and metabolic alterations in mice lacking β1- and β2-adrenergic receptors. Trends Cardiovasc Med. 2002;12:287–294. - PubMed
-
- Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, et al. Differential cardioprotective/cardiotoxic effects mediated by β-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. - PubMed
-
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different α2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. - PubMed
-
- Brum PC, Kosek J, Patterson A, Bernstein D, Kobilka B. Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol. 2002;283:H1838–H1845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

