Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice
- PMID: 18192436
- PMCID: PMC2254934
- DOI: 10.1105/tpc.107.055657
Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice
Abstract
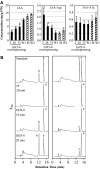
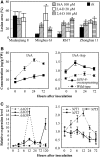
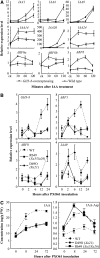
New evidence suggests a role for the plant growth hormone auxin in pathogenesis and disease resistance. Bacterial infection induces the accumulation of indole-3-acetic acid (IAA), the major type of auxin, in rice (Oryza sativa). IAA induces the expression of expansins, proteins that loosen the cell wall. Loosening the cell wall is key for plant growth but may also make the plant vulnerable to biotic intruders. Here, we report that rice GH3-8, an auxin-responsive gene functioning in auxin-dependent development, activates disease resistance in a salicylic acid signaling- and jasmonic acid signaling-independent pathway. GH3-8 encodes an IAA-amino synthetase that prevents free IAA accumulation. Overexpression of GH3-8 results in enhanced disease resistance to the rice pathogen Xanthomonas oryzae pv oryzae. This resistance is independent of jasmonic acid and salicylic acid signaling. Overexpression of GH3-8 also causes abnormal plant morphology and retarded growth and development. Both enhanced resistance and abnormal development may be caused by inhibition of the expression of expansins via suppressed auxin signaling.
Figures

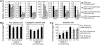

Similar articles
-
Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice.Plant Physiol. 2011 Jan;155(1):589-602. doi: 10.1104/pp.110.163774. Epub 2010 Nov 11. Plant Physiol. 2011. PMID: 21071600 Free PMC article.
-
The group I GH3 family genes encoding JA-Ile synthetase act as positive regulator in the resistance of rice to Xanthomonas oryzae pv. oryzae.Biochem Biophys Res Commun. 2019 Jan 22;508(4):1062-1066. doi: 10.1016/j.bbrc.2018.12.057. Epub 2018 Dec 13. Biochem Biophys Res Commun. 2019. PMID: 30553449
-
The Systemic Acquired Resistance Regulator OsNPR1 Attenuates Growth by Repressing Auxin Signaling through Promoting IAA-Amido Synthase Expression.Plant Physiol. 2016 Sep;172(1):546-58. doi: 10.1104/pp.16.00129. Epub 2016 Jul 4. Plant Physiol. 2016. PMID: 27378815 Free PMC article.
-
The Role of Plant Hormones in the Interaction of Colletotrichum Species with Their Host Plants.Int J Mol Sci. 2021 Nov 18;22(22):12454. doi: 10.3390/ijms222212454. Int J Mol Sci. 2021. PMID: 34830343 Free PMC article. Review.
-
Connecting primary and specialized metabolism: Amino acid conjugation of phytohormones by GRETCHEN HAGEN 3 (GH3) acyl acid amido synthetases.Curr Opin Plant Biol. 2022 Apr;66:102194. doi: 10.1016/j.pbi.2022.102194. Epub 2022 Feb 23. Curr Opin Plant Biol. 2022. PMID: 35219141 Review.
Cited by
-
Transcriptome Profiling of Huanglongbing (HLB) Tolerant and Susceptible Citrus Plants Reveals the Role of Basal Resistance in HLB Tolerance.Front Plant Sci. 2016 Jun 28;7:933. doi: 10.3389/fpls.2016.00933. eCollection 2016. Front Plant Sci. 2016. PMID: 27446161 Free PMC article.
-
Genome-wide analysis of the GH3 family in apple (Malus × domestica).BMC Genomics. 2013 May 2;14:297. doi: 10.1186/1471-2164-14-297. BMC Genomics. 2013. PMID: 23638690 Free PMC article.
-
Transcriptional profile of sweet orange in response to chitosan and salicylic acid.BMC Genomics. 2015 Apr 12;16(1):288. doi: 10.1186/s12864-015-1440-5. BMC Genomics. 2015. PMID: 25887907 Free PMC article.
-
Unveiling the Secretome of the Fungal Plant Pathogen Neofusicoccum parvum Induced by In Vitro Host Mimicry.J Fungi (Basel). 2022 Sep 17;8(9):971. doi: 10.3390/jof8090971. J Fungi (Basel). 2022. PMID: 36135697 Free PMC article.
-
Do trees grow on money? Auxin as the currency of the cellular economy.Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a001420. doi: 10.1101/cshperspect.a001420. Cold Spring Harb Perspect Biol. 2010. PMID: 20182619 Free PMC article. Review.
References
-
- Asai, T., Tena, G., Plotnikova, J., Willmann, M.R., Chiu, W.L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983. - PubMed
-
- Balestrini, R., Cosgrove, D.J., and Bonfante, P. (2005). Differential location of alpha-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta 220 889–899. - PubMed
-
- Bartling, D., Seedorf, M., Mithofer, A., and Weiler, E.W. (1992). Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur. J. Biochem. 205 417–424. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

