Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes
- PMID: 18188447
- PMCID: PMC2176194
- DOI: 10.1172/JCI34239
Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes
Erratum in
- J Clin Invest. 2009 Feb;119(2):421. Kanaho, Yasunori [added]
Abstract
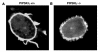
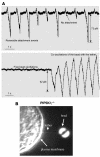
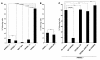
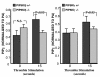
Phosphatidylinositol-4,5-bisphosphate (PIP(2)) is an abundant phospholipid that contributes to second messenger formation and has also been shown to contribute to the regulation of cytoskeletal dynamics in all eukaryotic cells. Although the alpha, beta, and gamma isoforms of phosphatidylinositol-4-phosphate-5-kinase I (PIP5KI) all synthesize PIP2, mammalian cells usually contain more than one PIP5KI isoform. This raises the question of whether different isoforms of PIP5KI fulfill different functions. Given the speculated role of PIP(2) in platelet and megakaryocyte actin dynamics, we analyzed murine megakaryocytes lacking individual PIP5KI isoforms. PIP5KIgamma(-/-) megakaryocytes exhibited plasma membrane blebbing accompanied by a decreased association of the membrane with the cytoskeleton. This membrane defect was rescued by adding back wild-type PIP5KIgamma, but not by adding a catalytically inactive mutant or a splice variant lacking the talin-binding motif. Notably, both PIP5KIbeta- and PIP5KIgamma(-/-) cells had impaired PIP(2) synthesis. However, PIP5KIbeta-null cells lacked the membrane-cytoskeleton defect. Furthermore, overexpressing PIP5KIbeta in PIP5KIgamma(-/-) cells failed to revert this defect. Megakaryocytes lacking the PIP5KIgamma-binding partner, talin1, mimicked the membrane-cytoskeleton defect phenotype seen in PIP5KIgamma(-/-) cells. These findings demonstrate a unique role for PIP5KIgamma in the anchoring of the cell membrane to the cytoskeleton in megakaryocytes, probably through a pathway involving talin. These observations further demonstrate that individual PIP5KI isoforms fulfill distinct functions within cells.
Figures
Similar articles
-
Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation.Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14064-9. doi: 10.1073/pnas.0804139105. Epub 2008 Sep 4. Proc Natl Acad Sci U S A. 2008. PMID: 18772378 Free PMC article.
-
Platelets lacking PIP5KIγ have normal integrin activation but impaired cytoskeletal-membrane integrity and adhesion.Blood. 2013 Apr 4;121(14):2743-52. doi: 10.1182/blood-2012-07-445205. Epub 2013 Jan 31. Blood. 2013. PMID: 23372168 Free PMC article.
-
Phosphatidylinositol phosphate 5-kinase Ibeta recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis.J Cell Biol. 2003 Aug 18;162(4):693-701. doi: 10.1083/jcb.200302051. Epub 2003 Aug 11. J Cell Biol. 2003. PMID: 12913109 Free PMC article.
-
One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane.Cell Mol Life Sci. 2010 Dec;67(23):3927-46. doi: 10.1007/s00018-010-0432-5. Epub 2010 Jun 18. Cell Mol Life Sci. 2010. PMID: 20559679 Free PMC article. Review.
-
Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton.Subcell Biochem. 2012;59:177-215. doi: 10.1007/978-94-007-3015-1_6. Subcell Biochem. 2012. PMID: 22374091 Review.
Cited by
-
The phosphatidylinositol-5' phosphatase synaptojanin1 limits integrin-mediated invasion of Staphylococcus aureus.Microbiol Spectr. 2024 Apr 2;12(4):e0200623. doi: 10.1128/spectrum.02006-23. Epub 2024 Feb 15. Microbiol Spectr. 2024. PMID: 38358281 Free PMC article.
-
Structural insights into lethal contractural syndrome type 3 (LCCS3) caused by a missense mutation of PIP5Kγ.Biochem J. 2018 Jul 26;475(14):2257-2269. doi: 10.1042/BCJ20180326. Biochem J. 2018. PMID: 29959184 Free PMC article.
-
Phosphatidylinositol-4-phosphate 5-kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-plexin-D1 through Arf6 protein.J Biol Chem. 2011 Sep 30;286(39):34335-45. doi: 10.1074/jbc.M111.259499. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795701 Free PMC article.
-
PIP kinases define PI4,5P₂signaling specificity by association with effectors.Biochim Biophys Acta. 2015 Jun;1851(6):711-23. doi: 10.1016/j.bbalip.2015.01.009. Epub 2015 Jan 21. Biochim Biophys Acta. 2015. PMID: 25617736 Free PMC article. Review.
-
Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation.Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14064-9. doi: 10.1073/pnas.0804139105. Epub 2008 Sep 4. Proc Natl Acad Sci U S A. 2008. PMID: 18772378 Free PMC article.
References
-
- Hokin M.R., Hokin L.E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J. Biol. Chem. 1953;203:967–977. - PubMed
-
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Cell Biol. 1998;10:254–261. - PubMed
-
- Doughman R.L., Firestone A.J., Anderson R.A. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 2003;194:77–89. - PubMed
-
- Oude Weernink P.A., Schmidt M., Jakobs K.H. Regulation and cellular roles of phosphoinositide 5-kinases. Eur. J. Pharmacol. 2004;500:87–99. - PubMed
-
- Malm B., Larsson H., Lindberg U. The profilin--actin complex: further characterization of profilin and studies on the stability of the complex. J. Muscle Res. Cell Motil. 1983;4:569–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

