PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis
- PMID: 18184860
- PMCID: PMC2396746
- DOI: 10.1681/ASN.2007030290
PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis
Abstract
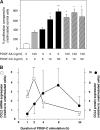
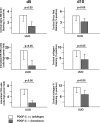
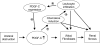
PDGF-C is a potent mitogen for fibroblasts in vitro. Transgenic PDGF-C overexpression in the heart or liver induces organ fibrosis, and PDGF-C expression is upregulated at sites of interstitial fibrosis in human and rat kidneys; however, the effect of inhibiting PDGF-C on the development of renal fibrosis in vivo is unknown. Renal fibrosis was induced in C57BL/6 mice by unilateral ureteral obstruction (UUO), and then mice were treated with neutralizing anti–PDGF-C antiserum or nonspecific IgG. An increase in PDGF-C expression was observed in fibrotic areas after UUO, contributed in large part by infiltrating macrophages. Treatment with anti–PDGF-C reduced renal fibrosis by 30% at day 5 and reduced interstitial myofibroblast accumulation by 57%. In vitro, PDGF-C was a potent mitogen for renal fibroblasts and induced chemokine expression. In vivo, anti–PDGF-C treatment produced a decrease in the expression of the renal chemokines CCL2 and CCL5 (85 and 67% reductions, respectively), accompanied by a significant decrease in leukocyte infiltration and CCR2 mRNA expression. Further supporting a role of PDGF-C in renal fibrosis, PDGF-C−/− mice demonstrated a reduction in fibrosis and leukocyte infiltration in response to UUO compared with wild-type littermates. In conclusion, specific neutralization or lack of PDGF-C reduces the development of renal inflammation and fibrosis in obstructed mouse kidneys. Leukocyte-derived PDGF-C induces chemokine expression, which may lead to the recruitment of additional leukocytes, creating an amplification loop for renal inflammation and fibrosis.
Figures




Similar articles
-
Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis.Am J Pathol. 2013 Jan;182(1):107-17. doi: 10.1016/j.ajpath.2012.09.006. Epub 2012 Nov 7. Am J Pathol. 2013. PMID: 23141925
-
Complement C5 mediates experimental tubulointerstitial fibrosis.J Am Soc Nephrol. 2007 May;18(5):1508-15. doi: 10.1681/ASN.2006121343. Epub 2007 Mar 27. J Am Soc Nephrol. 2007. PMID: 17389734
-
Role of platelet-derived growth factor-CC in capillary rarefaction in renal fibrosis.Am J Pathol. 2015 Aug;185(8):2132-42. doi: 10.1016/j.ajpath.2015.04.022. Am J Pathol. 2015. PMID: 26216283
-
Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family.FEBS J. 2005 Nov;272(22):5723-41. doi: 10.1111/j.1742-4658.2005.04989.x. FEBS J. 2005. PMID: 16279938 Review.
-
Role of PDGF in fibrotic diseases and systemic sclerosis.Rheumatology (Oxford). 2008 Oct;47 Suppl 5:v2-4. doi: 10.1093/rheumatology/ken265. Rheumatology (Oxford). 2008. PMID: 18784131 Review.
Cited by
-
The pathogenic role of succinate-SUCNR1: a critical function that induces renal fibrosis via M2 macrophage.Cell Commun Signal. 2024 Jan 30;22(1):78. doi: 10.1186/s12964-024-01481-5. Cell Commun Signal. 2024. PMID: 38291510 Free PMC article.
-
Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis.Am J Pathol. 2011 Feb;178(2):911-23. doi: 10.1016/j.ajpath.2010.10.012. Am J Pathol. 2011. PMID: 21281822 Free PMC article.
-
Lung Cancer Cell-Derived Secretome Mediates Paraneoplastic Inflammation and Fibrosis in Kidney in Mice.Cancers (Basel). 2020 Nov 28;12(12):3561. doi: 10.3390/cancers12123561. Cancers (Basel). 2020. PMID: 33260558 Free PMC article.
-
Identification of CD2, CCL5 and CCR5 as potential therapeutic target genes for renal interstitial fibrosis.Ann Transl Med. 2019 Sep;7(18):454. doi: 10.21037/atm.2019.08.62. Ann Transl Med. 2019. PMID: 31700890 Free PMC article.
-
Age-associated microenvironmental changes highlight the role of PDGF-C in ER+ breast cancer metastatic relapse.Nat Cancer. 2023 Apr;4(4):468-484. doi: 10.1038/s43018-023-00525-y. Epub 2023 Mar 13. Nat Cancer. 2023. PMID: 36914817 Free PMC article.
References
-
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U: PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2: 302–309, 2000 - PubMed
-
- Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, Gao Z, Shoemaker K, Bukowski TR, Moore M, Feldhaus AL, Humes JM, Palmer TE, Hart CE: Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem 276: 27406–27414, 2001 - PubMed
-
- Zhuo Y, Hoyle GW, Shan B, Levy DR, Lasky JA: Over-expression of PDGF-C using a lung specific promoter results in abnormal lung development. Transgenic Res 15: 543–555, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

