A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages
- PMID: 18183290
- PMCID: PMC2169301
- DOI: 10.1371/journal.pone.0001409
A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages
Abstract
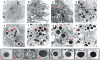
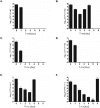
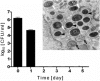
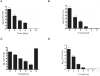
Although considered to be an extracellular pathogen, Staphylococcus aureus is able to invade a variety of mammalian, non-professional phagocytes and can also survive engulfment by professional phagocytes such as neutrophils and monocytes. In both of these cell types S. aureus promptly escapes from the endosomes/phagosomes and proliferates within the cytoplasm, which quickly leads to host cell death. In this report we show that S. aureus interacted with human monocyte-derived macrophages in a very different way to those of other mammalian cells. Upon phagocytosis by macrophages, S. aureus persisted intracellularly in vacuoles for 3-4 days before escaping into the cytoplasm and causing host cell lysis. Until the point of host cell lysis the infected macrophages showed no signs of apoptosis or necrosis and were functional. They were able to eliminate intracellular staphylococci if prestimulated with interferon-gamma at concentrations equivalent to human therapeutic doses. S. aureus survival was dependent on the alternative sigma factor B as well as the global regulator agr, but not SarA. Furthermore, isogenic mutants deficient in alpha-toxin, the metalloprotease aureolysin, protein A, and sortase A were efficiently killed by macrophages upon phagocytosis, although with different kinetics. In particular alpha-toxin was a key effector molecule that was essential for S. aureus intracellular survival in macrophages. Together, our data indicate that the ability of S. aureus to survive phagocytosis by macrophages is determined by multiple virulence factors in a way that differs considerably from its interactions with other cell types. S. aureus persists inside macrophages for several days without affecting the viability of these mobile cells which may serve as vehicles for the dissemination of infection.
Conflict of interest statement
Figures
Similar articles
-
Inside job: Staphylococcus aureus host-pathogen interactions.Int J Med Microbiol. 2018 Aug;308(6):607-624. doi: 10.1016/j.ijmm.2017.11.009. Epub 2017 Nov 26. Int J Med Microbiol. 2018. PMID: 29217333 Review.
-
The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils.Autophagy. 2021 Apr;17(4):888-902. doi: 10.1080/15548627.2020.1739443. Epub 2020 Mar 15. Autophagy. 2021. PMID: 32174246 Free PMC article.
-
Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages.Cell Microbiol. 2016 Aug;18(8):1172-83. doi: 10.1111/cmi.12577. Epub 2016 Mar 18. Cell Microbiol. 2016. PMID: 26895738
-
Recognition of apoptotic cells by human peripheral blood monocytes does not alter their ability to phagocytize and kill Staphylococcus aureus.Arch Immunol Ther Exp (Warsz). 2004 Jan-Feb;52(1):50-8. Arch Immunol Ther Exp (Warsz). 2004. PMID: 15053233
-
Intracellular Staphylococcus aureus: live-in and let die.Front Cell Infect Microbiol. 2012 Apr 24;2:43. doi: 10.3389/fcimb.2012.00043. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919634 Free PMC article. Review.
Cited by
-
Staphylococcus aureus Exploits a Non-ribosomal Cyclic Dipeptide to Modulate Survival within Epithelial Cells and Phagocytes.PLoS Pathog. 2016 Sep 15;12(9):e1005857. doi: 10.1371/journal.ppat.1005857. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27632173 Free PMC article.
-
Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus.Microbes Infect. 2012 Sep;14(11):989-99. doi: 10.1016/j.micinf.2012.04.013. Epub 2012 May 2. Microbes Infect. 2012. PMID: 22613209 Free PMC article.
-
Staphylococcus aureus Induces IFN-β Production via a CARMA3-Independent Mechanism.Pathogens. 2021 Mar 4;10(3):300. doi: 10.3390/pathogens10030300. Pathogens. 2021. PMID: 33806598 Free PMC article.
-
An immobilized liquid interface prevents device associated bacterial infection in vivo.Biomaterials. 2017 Jan;113:80-92. doi: 10.1016/j.biomaterials.2016.09.028. Epub 2016 Sep 30. Biomaterials. 2017. PMID: 27810644 Free PMC article.
-
Measurement of Accumulation of Antibiotics to Staphylococcus aureus in Phagosomes of Live Macrophages.Angew Chem Int Ed Engl. 2024 Jan 15;63(3):e202313870. doi: 10.1002/anie.202313870. Epub 2023 Dec 15. Angew Chem Int Ed Engl. 2024. PMID: 38051128 Free PMC article.
References
-
- Archer GL. Staphylococcus aureus: A well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. - PubMed
-
- Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. - PubMed
-
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant: an emerging threat. Lancet Infect Dis. 2005;5:275–286. - PubMed
-
- Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. - PubMed
-
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

