Neuroimmune mechanisms in patients with atopic dermatitis during chronic stress
- PMID: 18181968
- PMCID: PMC2229631
- DOI: 10.1111/j.1468-3083.2007.02202.x
Neuroimmune mechanisms in patients with atopic dermatitis during chronic stress
Abstract
Objective: To identify pathoaetiological neuroimmune mechanisms in patients with atopic dermatitis (AD) and chronic stress, focusing at nerve density, sensory neuropeptides, and the serotonergic system.
Methods: Eleven patients with AD with histories of stress worsening were included. Biopsies from involved and non-involved skin were processed for immunohistochemistry. Salivary cortisol test was done as a marker for chronic stress.
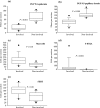
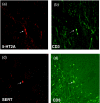
Results: There were more acanthosis and fewer nerve fibres in epidermis and papillary dermis of involved compared with non-involved skin. Whereas there was no significant change in the number of substance P and calcitonin gene-related peptide-positive nerve fibres between the involved and non-involved skin, there was an increase in the epidermal fraction of 5-hydroxtrytamine 1A (5-HT1A) receptor and serotonin transporter protein (SERT) immunoreactivity in the involved skin. The number of 5-HT2AR, CD3-positive cells, and SERT-positive cells, most of them being CD3 positive, was increased in involved skin. There was an increase in mast cells in the involved skin, and these cells were often located close to the basement membrane. There was a strong tendency to a correlation between 5-HT2AR positive cells in the papillary dermis of involved skin and low cortisol ratios, being an indicator of chronic stress.
Conclusion: A changed innervation and modulation of the serotonergic system are indicated in chronic atopic eczema also during chronic stress.
Figures

Similar articles
-
Effect of chronic mild stress on serotonergic markers in the skin and brain of the NC/Nga atopic-like mouse strain.Arch Dermatol Res. 2011 Nov;303(9):625-33. doi: 10.1007/s00403-011-1138-8. Epub 2011 Mar 15. Arch Dermatol Res. 2011. PMID: 21400247
-
Serotonergic Markers in Atopic Dermatitis.Acta Derm Venereol. 2016 Aug 23;96(6):732-6. doi: 10.2340/00015555-2354. Acta Derm Venereol. 2016. PMID: 26831833
-
Atopic dermatitis, stinging, and effects of chronic stress: a pathocausal study.J Am Acad Dermatol. 2004 Dec;51(6):899-905. doi: 10.1016/j.jaad.2004.05.035. J Am Acad Dermatol. 2004. PMID: 15583580
-
Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates.Acta Derm Venereol. 2012 Jan;92(1):7-15. doi: 10.2340/00015555-1188. Acta Derm Venereol. 2012. PMID: 22101513 Free PMC article. Review.
-
Atopic dermatitis: allergic dermatitis or neuroimmune dermatitis?An Bras Dermatol. 2016 Jul-Aug;91(4):479-88. doi: 10.1590/abd1806-4841.20164211. An Bras Dermatol. 2016. PMID: 27579744 Free PMC article. Review.
Cited by
-
Skin and Psychosomatics - Psychodermatology today.J Dtsch Dermatol Ges. 2020 Nov;18(11):1280-1298. doi: 10.1111/ddg.14328. J Dtsch Dermatol Ges. 2020. PMID: 33251751 Free PMC article.
-
Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system.Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1-115. doi: 10.1007/978-3-642-19683-6_1. Adv Anat Embryol Cell Biol. 2012. PMID: 22894052 Free PMC article. Review.
-
Mass quarantine measures in the time of COVID-19 pandemic: psychosocial implications for chronic skin conditions and a call for qualitative studies.J Eur Acad Dermatol Venereol. 2020 Jul;34(7):e293-e294. doi: 10.1111/jdv.16535. Epub 2020 May 15. J Eur Acad Dermatol Venereol. 2020. PMID: 32330329 Free PMC article. No abstract available.
-
Atopic Dermatitis and Comorbidity.Healthcare (Basel). 2020 Mar 25;8(2):70. doi: 10.3390/healthcare8020070. Healthcare (Basel). 2020. PMID: 32218222 Free PMC article.
-
Immunohistochemical Evaluation of Role of Serotonin in Pathogenesis of Psoriasis.J Clin Diagn Res. 2016 Oct;10(10):EC05-EC09. doi: 10.7860/JCDR/2016/22692.8719. Epub 2016 Oct 1. J Clin Diagn Res. 2016. PMID: 27891342 Free PMC article.
References
-
- Leung DYM, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. - PubMed
-
- Schultz Larsen F. Atopic dermatitis: a genetic-epidemiologic study in a population-based twin sample. J Am Acad Dermatol. 1993;28:719–723. - PubMed
-
- Pastar Z, Lipozencic J, Ljubojevic S. Etiopathogenesis of atopic dermatitis – an overview. Acta Dermatovenerol Croat. 2005;13:54–62. - PubMed
-
- Morren MA, Przybilla B, Bamelis M, Heykants B, Reynaers A, Degreef H. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

